There are currently 118 elements listed in the periodic table. Superheavy elements (SHE) are elements with proton numbers (Z) of 104 or greater. These elements are produced by fusing atomic nuclei of lighter elements using particle accelerators. The GSI Helmholtz Center for Heavy Ion Research in Darmstadt, Germany, created elements 107 through 112 by bombarding elements 82 (lead) and 83 (bismuth) with moderately heavy projectile nuclei (Z = 24, 26, 28, and 30).
Professor Michael Block, GSI Darmstadt, and University of Mainz, Germany, and his team measure atomic and nuclear properties of the heaviest elements. He and his team were the first to determine the size and shape of nobelium (Z = 102) atoms through laser spectroscopy.
What fascinates you about working with heavy elements?
What I find personally exciting are basic questions such as: Where are the limits of nuclear stability? What is the heaviest element? But, of course, the experimental appeal is also fascinating, namely, to push the measurements and the methods to the limits of what is feasible and to develop them further in such a way that these limits are pushed further and further out.
When I started here at GSI, we measured masses of atomic nuclei in the range of tin, holmium, or dysprosium. So rather light to medium elements from today’s perspective. The production rates were relatively high – maybe a factor of 1000 higher than for the elements we measure today. However, these measurements were already very difficult at that time.
At that time, I didn’t think that we would be able to measure masses of heavy elements with our technique at all. Meanwhile, nobelium (Z = 102), which we measured here for the first time in 2008, is our test ion with which we optimize everything. Today we can push down the rates compared to the first nobelium experiments by a factor of 100 and still make a measurement.
Last year in June/July, for example, we made a measurement where we only detected an average of three atoms per day on our detector. This still allowed us to determine the mass of the atom to an accuracy of 10–8 or 10–9. This is an incredibly big challenge from the technology point of view. And at the moment nobody else in the world can measure the masses of such rare and heavy nuclei with such accuracy. I find it very exciting to continuously improve the performance of the method over the years.
Another aspect that fascinates me, and that I am trying to push in my group, is that we obtain as much information as possible from a single experiment. Of course, it’s nice to create a new element and prove its existence (unambiguously). However, actually, we would like to be able not just to prove the existence but to measure several properties of a new element, because in the end, we want to understand why one element is longer-lived than another, or why a certain combination of protons and neutrons is more stable. We really want to measure the atomic, the nuclear, and, in the end, the chemical properties in as many different ways as possible by using different techniques to then put everything together in a larger picture.
So how do you measure the atomic radius, for example?
With laser spectroscopy, we can measure the orbit of an electron moving around an atomic nucleus with very high accuracy. From this, we can obtain the charge radius of a nucleus, and, hence, see how the nucleus is shaped or how large the nucleus is.
Can you please explain this in more detail?
We have a positively charged atomic nucleus and a negatively charged electron interacting with each other. If you look from far away, the nucleus looks like a point-like charge distribution. But if you take a closer look, you will see that the nucleus, of course, is not a point but something larger. And many of the atomic nuclei are not spheres. Based on experimental observations and theoretical predictions, we know that only every third atomic nucleus is really spherical; the Pb-208 nucleus is an example. Most atomic nuclei are deformed.
If the nucleus is deformed, for example, elongated, cigar-shaped, or American football-shaped, the charge is also distributed over a different volume. If you move your electron around it, you have a place where the charge is a little further away and where it is a little closer. In principle, this leads to small changes in the electron’s orbit. If you measure these changes very precisely, you can use the electron as a sensor to measure the nucleus and its charge distribution. There you learn something about the size of the atomic nucleus, such as its radius, and about its shape.
And you can do that with superheavy elements?
Yes. We recently did it for element 108 [nobelium]. We hope we can do that with even heavier elements in the future. At 102 the production rates are in the order of one atom per second to one atom per minute. For much heavier elements we have to deal with a rate that is lower by a factor of 1000. But when you’ve made it once, it’s not completely illusory.
With a production rate of one atom per minute and small half-lives, how do you manage to hit an electron of such an atom?
We slow down the atom in a cell filled with gas.
First, we create nobelium isotopes by fusing calcium ions with lead nuclei. The formed nobelium atoms then move at about 10 % of the speed of light. We let them fly through a thin foil into a cell filled with argon gas. The gas slows down the atoms. With the help of an electric field, they are collected on a tantalum wire. Then we know where the particle sits until it decays.
We then heat up the wire. The atoms simply evaporate. Of course, this atomic cloud moves a little, but that’s only a few millimeters. Then we shoot laser radiation into this area. If we do it right, we hit the atom. When we hit the atom with two laser pulses, the atom is ionized and can be transported to a detector that registers its radioactive decay. If we vary the frequency, i.e., the color, of the laser light we can record the number of decays for a different frequency and get a curve. This allows us to measure the distance of the electron from the nucleus.
We did that for three different isotopes of nobelium (see Fig. 1). That means we have 102 protons and 152, 153 or 154 neutrons. If I take two neutrons from the nucleus, I still have the same number of positively charged particles, but they are arranged differently. The charge distribution is changing. This can be seen in Figure 1.
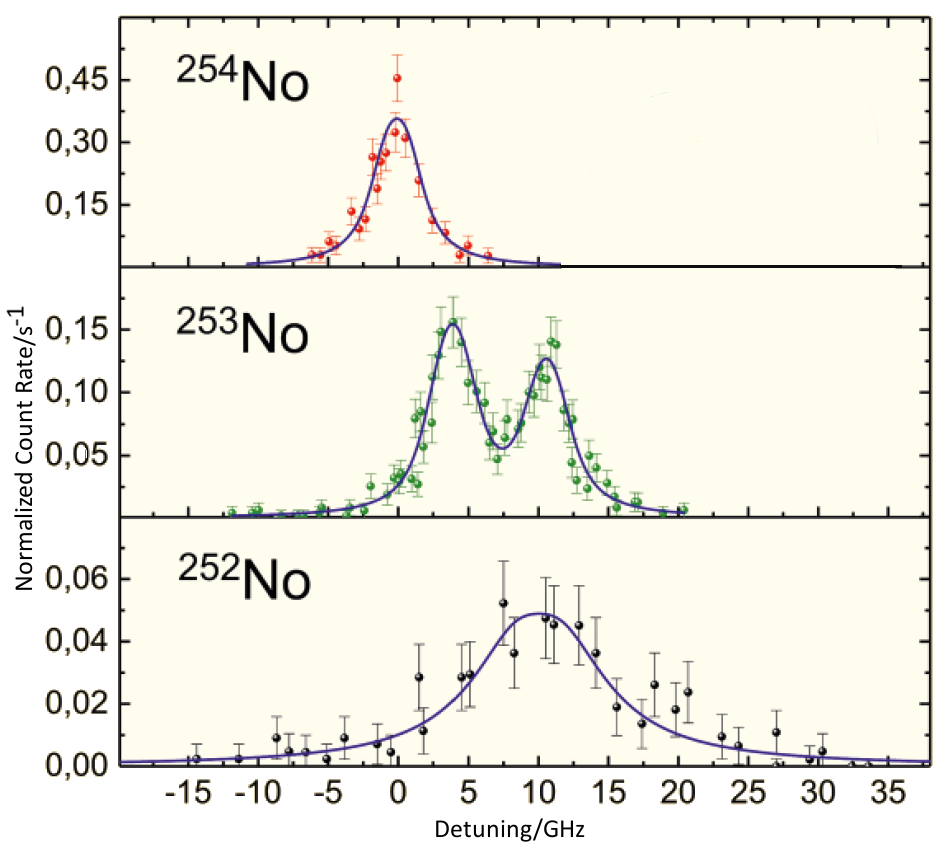
Figure 1. Measured excitation spectra for the isotopes No-252, No-253, and No-254. The spectrum of the No-253 isotope shows a hyperfine structure splitting [1].
As Figure 2 shows, the actual shape of the charge distribution is not round, but elongated, similar to the shape of a lemon. If it were a homogeneous distribution, the inner part would have to be dark red. But because the middle is yellow again, here you have a so-called small dip or a central depression. Sometimes this is also called a bubble nucleus.
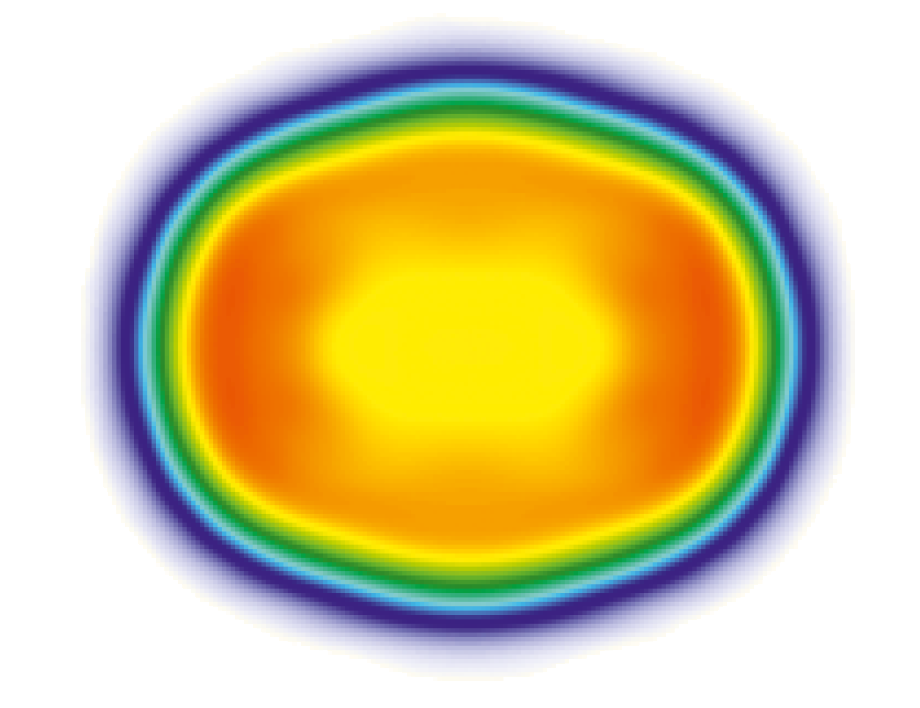
Figure 2. Calculated charge distribution for the nucleus of the isotope 254-No with a significantly reduced charge density in the center of the nucleus [1].
With the heavy elements, this dip is caused by the very strong repulsion of the protons to each other. It pushes the positive charges a little out of the center, which produces such a small dip, a reduced charge density.
It’s exciting to see how this reduced charge density develops with heavier elements.
Exactly. With nobelium, it’s just a few percents. If the theoretical models are correct, then for an element like oganesson (Z = 118) this is perhaps already 20 or 30 %. In the case of atoms that have not yet been discovered, it can really be the case that a really large dip would be in the middle.
What else do you measure?
The same technique also allows us to lift an electron from one orbit to the next, so you excite the electron and measure the transition. This allows us to measure the energy of different electronic levels. If you do this with high precision for many high-lying levels, you can determine the ionization potential, for example, and see how much energy you need to extract an electron from nobelium. This is the transition from (atomic) physics to chemistry.
The difficulty at the beginning – three, four, five years ago, when we started with these experiments – was that we didn’t know in which frequencies the lines actually lie. The uncertainty of the predicted values required us to scan the laser frequency in about 3,000 steps to find the resonance.
You also measure the mass of heavy atomic nuclei. How do you do that?
To do this, the particle is stored in a magnetic field within a Penning trap. A charged particle in a magnetic field makes a circular motion, also called cyclotron motion. If the particle can move up or down, it would make a kind of corkscrew path. To prevent this, we put an electrode structure over it.
The circular motion is imaged on an ion detector. This allows us to determine the frequency at which the ions circulate in the Penning trap. The frequency depends on the charge state, the magnetic field strength, and the mass. In principle, you can use it to weigh the atoms. We did this since 2008 for many heavy elements – recently, in 2018, up to Rf.
How long does such a measurement take?
It depends on the production rate and the half-life of the ion. A typical measurement takes a few hours but sometimes it may take even a few days.
And what do you learn from measuring the mass?
An atom, nobelium, for example, has 102 protons, 152 neutrons, and 102 electrons. Of course, we know what an electron, a proton, and a neutron weigh. The atom is lighter than the sum of the masses of the individual constituent particles. This is because the energy stored in the bond of this system becomes visible as a missing mass. This is the so-called mass defect, which was experimentally proven at the end of the 1920s. This is typically an effect on the percent level. So it is not a small effect. It reflects the binding energy of the nucleus.
We measure the binding energy of an atom via its mass. We can then compare what happens if, for example, I look at the nobelium with 152 neutrons compared to the nobelium with 159 neutrons, or with 154. How does the binding energy change for nuclei with different numbers of protons and neutrons? We compare the mass or binding energy, say, for a nucleus with 102 protons with 104 or 100 for the same number of neutrons. Then you can find out which combination of protons and neutrons are bound the strongest. These are the ominous “magic numbers” that arise from closed nuclear shells, meaning closed proton and/or closed neutron shells.
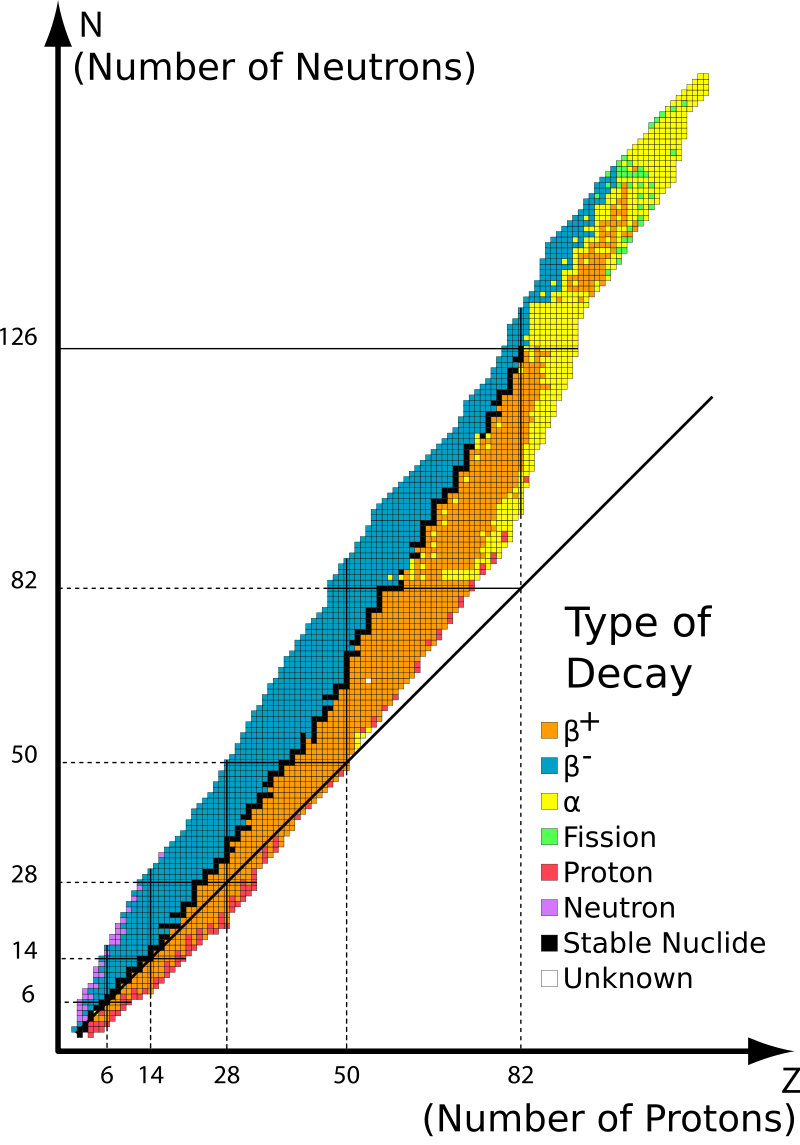
Figure 3. Map of nuclides.
In the chart of nuclides, the map in Figure 3, we plot all known nuclides with the proton number increasing from bottom to top and the neutron number increasing from left to right. Black squares represent stable or very long-lived isotopes. Colored squares indicate unstable, i.e., radioactive, nucleotides. The different colors represent different radioactive decay modes.
Now you can think about why there are so many stable isotopes in some places. For example, there are many stable isotopes of nickel, calcium, or tin.
Why is this?
The black lines indicate the magic numbers. Whenever magic numbers occur for either protons or neutrons or for both together, we have a closed shell in the nucleus. Whenever a shell is closed, you need extra energy to get a nucleon to the next higher one. That is why nuclei with closed shells are particularly stable. This has the effect, for example, that there are many stable isotopes of the same elements and not of others, or that some heavy elements live longer than others.
Theoretical measurements predict a so-called island of stability of superheavy elements with Z ≈ 114 and N ≈ 184. Elements in this region would be rather long-lived. However, this area is currently not experimentally accessible.
To experimentally find out where the magic numbers are located, we can, for example, perform mass measurements and compare the resulting binding energies for different isotope combinations with each other.
So mass and atomic radius measurements are your key experiments?
Exactly. Of course, we also do classical (nuclear) decay spectroscopy, where we study the radioactive decays in detail. In the future, we will also carry out measurements where we transport ions through gases and determine ion mobilities. But laser spectroscopy and mass measurements are presently the two key experiments that have led to the greatest progress in the recent past.
Before 2008, there were no mass measurements above Z = 100 at all. Thanks to our activities, there are a few now. The same holds for our laser spectroscopy experiments in which we entered the region of Z = 100 in 2015.
What is the reason for this?
Up to fermium (Z = 100), you can in principle still produce the elements in “large” quantities in the reactor, sometimes just a picogram or nanogram. Then you can chemically separate the element, put it in a transport package, send it by post, and then you can make such measurement at home in your laboratory. This is no longer possible for elements above fermium. You have to create them at the particle accelerator. This, of course, involves lower production rates and typically shorter half-lives. This makes the measurements much more difficult.
We carried out such mass measurements on nobelium for the first time in 2008. In 2018, we have extended these measurements up to rutherfordium (Z = 104). Above rutherfordium, such measurements have not yet been carried out. We are still the only group worldwide who can perform mass measurements on very rare elements with such high precision. Recently, a Japanese group has made a first measurement on the nobelium isotope, which we studied in 2008 but with a different technique that is typically less precise.
How long does it take to develop such a method?
It can take several years. The basic method itself already existed, but then it had to be adapted so that it also worked for nobelium. This took several years. We really developed this especially for this experiment, including the whole setup we have for it.
How do you further develop a method? You certainly have measuring times and it is certainly not very convenient to do such an experiment. Surely you can’t just change these and those parameters and see what happens?
Exactly. On the one hand, you can first go to the lighter homologues and assume that the heavy element will behave similarly in terms of atomic and chemical properties.
For example, for the laster spectroscopy, you could use ytterbium instead of nobelium. First, you practice a bit with the ytterbium and see how all the processes work. But in the end, of course, there are some steps that really only work with nobelium. For example, at what temperature do the nobelium atoms evaporate from the tantalum wire? We really had to measure that. We didn’t know that before. At best, there are predictions. But they are often not accurate enough for our needs. You always have to keep in mind that these are high-precision measurements.
Can you do something like that in one measuring time?
In the case of nobelium, it actually took three measurement times. In the very first experiment, we were all very optimistic. I think that was in 2006. We thought we could do the full measurement in two days of experiment time. In 2014 we tried it again, and in 2015, it worked for the first time. Then we improved our setup and in 2016 we made several measurements on three different nobelium isotopes. In March we tried to take it a little further.
Nobelium-152 has a half-life of about 2.5 s. This makes the measurements even more difficult.
It also takes a while from the conception of the experiment, to the construction of the apparatus, and the test measurements. We got a measurement time this year and if something doesn’t work out, we have to come back next year. In this respect, everything has to fit in with the measurement time.
The other big difficulty with these experiments is that you have to have patience. The actual measurements take a very long time and the preparation is even longer. This is much more complex than many classical nuclear physics experiments. We cannot simply set up and optimize an established detector system, then chose the proper combination of projectile beam and target, produce the nuclei of interest, and collect data. Here, the entire method and setup had to be adapted to the specific requirements of nobelium and work even for the lowest production rates.
Is there a permanent team that works interdisciplinarily to ensure that everything fits together, or does the team reassemble at each measurement time?
It’s a mixture. We have a small group of permanent scientists who make sure that the entire know-how is available for years. This is complemented, for example, by postdocs who are available for two or three years and Ph.D. students who measure three or four years or whatever time is required for their doctoral theses. And then there are a few master’s students. In addition, there are some external partners. This means that there is a small core team that has the expertise and know-how. Development and measurement work are then outsourced to additional people. Typically, there are twelve, maybe up to 20 people.
During the measurement period, you have to supervise the experiment around the clock, usually in three shifts. You need at least two to three people per shift. We usually have small groups of four to five people in the sub-projects of our department. It is thus difficult to carry out such an experiment with a small group of people if it takes two to three weeks of measurement time and a few more weeks of preparation. You need a few more people. During the measuring times, colleagues from other groups also support you.
Very interesting. Thank you for giving us a tiny insight into your fascinating work on superheavy and very short-lived atoms.
Reference
[1] Sebastian Raeder, Mustapha Laatiaoui, Michael Block, Vermessung von Nobelium-Isotopen mit Laserlicht, Phys. Unserer Zeit 2018, 49, 214–215. https://doi.org/10.1002/piuz.201870504
Michael Block studied physics at the Johannes Gutenberg University, Mainz, Germany, until 1997. He gained his Ph.D. there in 2002, and performed a postdoc at GSI, Darmstadt. He became project leader of the SHIPTRAP experiment there, did a two-year research stay in the USA, and became deputy head of the Superheavy Elements Physics department at GSI. Since 2015, Michael Block has been a professor at the University of Mainz, Germany, and the head of the Superheavy Elements Physics department at the GSI Darmstadt and the Helmholtz-Institut Mainz (HIM) in Mainz, Germany.
Selected Awards
- Flerov Prize 2013
Selected Articles
- Schaleneffekte in den schwersten Elementen,
Michael Block, Lutz Schweikhard, Klaus Blaum,
Physik in unserer Zeit 2013, 44, 9–10.
https://doi.org/10.1002/piuz.201390014 - Island of Heavyweights,
Christoph E. Düllmann, Michael Block,
Scientific American 2018, 318, 46–53.
https://doi.org/10.1038/scientificamerican0318-46 - Direct mass measurements above uranium bridge the gap to the island of stability,
M. Block, D. Ackermann, K. Blaum, C. Droese, M. Dworschak, S. Eliseev, T. Fleckenstein, E. Haettner, F. Herfurth, F. P. Heßberger, S. Hofmann, J. Ketelaer, J. Ketter, H.-J. Kluge, G. Marx, M. Mazzocco, Yu. N. Novikov, W. R. Plaß, A. Popeko, S. Rahaman, D. Rodríguez, C. Scheidenberger, L. Schweikhard, P. G. Thirolf, G. K. Vorobyev, C. Weber,
Nature 2010, 463, 785–788.
https://doi.org/10.1038/NATURE08774 - Direct Mapping of Nuclear Shell Effects in the Heaviest Elements,
E. M. Ramirez, D. Ackermann, K. Blaum, M. Block, C. Droese, C. E. Dullmann, M. Dworschak, M. Eibach, S. Eliseev, E. Haettner, F. Herfurth, F. P. Hessberger, S. Hofmann, J. Ketelaer, G. Marx, M. Mazzocco, D. Nesterenko, Y. N. Novikov, W. R. Plass, D. Rodriguez, C. Scheidenberger, L. Schweikhard, P. G. Thirolf, C. Weber,
Science 2012, 337, 1207–1210.
https://doi.org/10.1126/SCIENCE.1225636 - Probing Sizes and Shapes of Nobelium Isotopes by Laser Spectroscopy,
S. Raeder, D. Ackermann, H. Backe, R. Beerwerth, J. C. Berengut, M. Block, A. Borschevsky, B. Cheal, P. Chhetri, Ch. E. Düllmann, V. A. Dzuba, E. Eliav, J. Even, R. Ferrer, V. V. Flambaum, S. Fritzsche, F. Giacoppo, S. Götz, F. P. Heßberger, M. Huyse, U. Kaldor, O. Kaleja, J. Khuyagbaatar, P. Kunz, M. Laatiaoui, F. Lautenschläger, W. Lauth, A. K. Mistry, E. Minaya Ramirez, W. Nazarewicz, S. G. Porsev, M. S. Safronova, U. I. Safronova, B. Schuetrumpf, P. Van Duppen, T. Walther, C. Wraith, A. Yakushev,
Physical Review Letters 2018.
https://doi.org/10.1103/PhysRevLett.120.232503 - Atom-at-a-time laser resonance ionization spectroscopy of nobelium,
Mustapha Laatiaoui, Werner Lauth, Hartmut Backe, Michael Block, Dieter Ackermann, Bradley Cheal, Premaditya Chhetri, Christoph Emanuel Düllmann, Piet van Duppen, Julia Even, Rafael Ferrer, Francesca Giacoppo, Stefan Götz, Fritz Peter Heßberger, Mark Huyse, Oliver Kaleja, Jadambaa Khuyagbaatar, Peter Kunz, Felix Lautenschläger, Andrew Kishor Mistry, Sebastian Raeder, Enrique Minaya Ramirez, Thomas Walther, Calvin Wraith, Alexander Yakushev,
Nature 2016, 538, 495–498.
https://doi.org/10.1038/nature19345