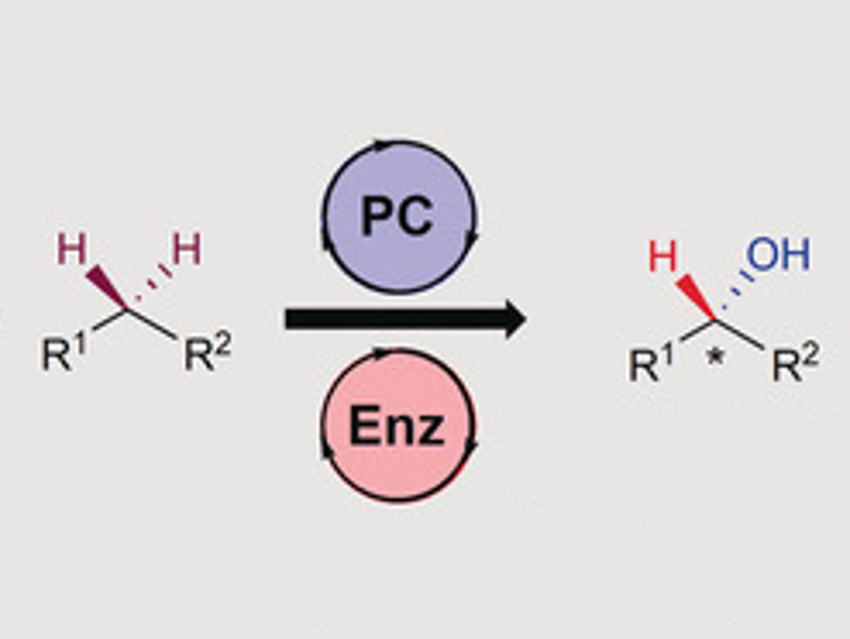
The activation of usually unreactive C–H bonds is of interest in organic chemistry. Many chemical products such as pharmaceuticals or fine chemicals are enantioenriched alcohols, which can be created by C−H hydroxylations. The chemical and pharmaceutical industries are especially interested in procedures that result in high enantioselectivities and high yields using mild conditions. Enzymes such as Cytochrome P450 often fulfill these requirements, but the resulting product can be hard to predict and differs by substrate class.
Karl A. Scheidt, Northwestern University, Evanston, IL, USA, and colleagues have found that combining enzymatic with photoredox catalysis can overcome these limitations for benzylic C–H bonds. With the help of oxygen, the benzylic C–H bond is transformed into a ketone via a peroxide intermediate. A ketoreductase enzyme then reduces the ketone selectively to the alcohol, using isopropyl alcohol as the hydride donor.
This procedure can be used for many different substrates with benzylic C–H bonds. Heteroaromatic compounds and allylic alcohols, however, did not react. The mild conditions at room temperature and the use of green solvents, as well as the high enantioselectivities make this procedure a possible candidate for an industrial process.
- Combined Photoredox/Enzymatic C−H Benzylic Hydroxylations,
Rick C. Betori, Catherine M. May, Karl A. Scheidt,
Angew. Chem. Int. Ed. 2019.
https://doi.org/10.1002/anie.201909426