Fluorescent molecules have been widely used in many areas of biological and medical research. Hiroyuki Kagechika, Tokyo Medical and Dental University (TMDU), Japan, Tomoya Hirano, Osaka University of Pharmaceutical Sciences, Japan, and colleagues have developed a fluorescent amino acid that can be incorporated into peptides. There, it allows the team to detect interactions between the resulting fluorescent peptide and calmodulin, a calcium-binding protein.
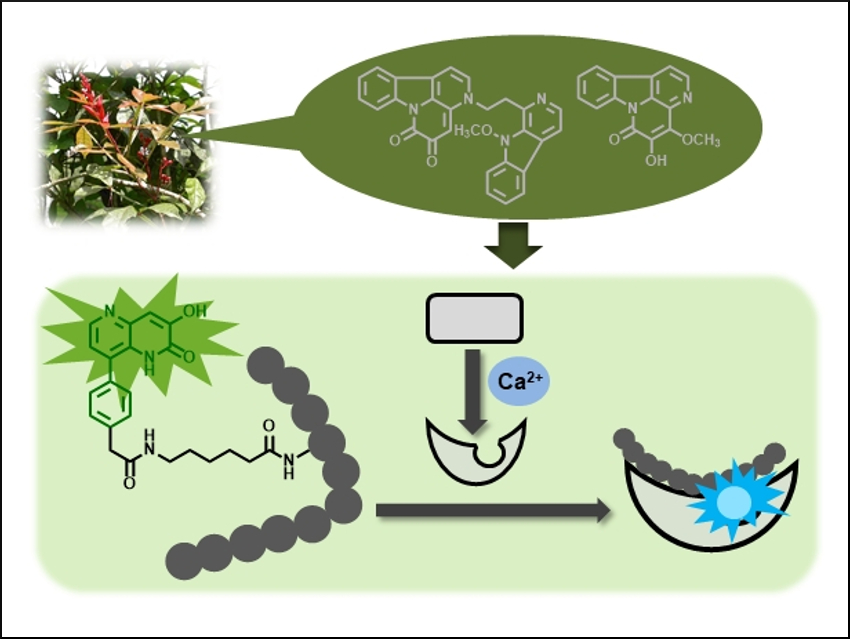
The researchers have synthesized fluorophores with a common structure, a 1,5-naphthyridin-2(1H)-one unit. This type of fluorophore is a structural component of the fluorescent natural compounds amarastelline A and nigakinone (pictured top). One of these fluorophores, the 3-hydroxy-8-phenyl derivative, shows a solvent-polarity-dependent change of its fluorescence ratio at 370/480 nm and strong fluorescence in both aqueous solutions and in less polar solvents.
To apply this property to the study of biomolecule interactions, the fluorophore was converted into an amino acid analogue and introduced into a C5 peptide (pictured bottom left). The fluorescence of the peptide could then be used to sense its interaction with calmodulin using fluorescent ratiometric measurements. The fluorescence ratio of the peptide was changed by the calmodulin only in the presence of Ca2+. This indicates that the fluorescent peptide can detect the conformational change of calmodulin induced by Ca2+ (pictured bottom right). These findings indicate the usefulness of natural compounds for the development of new fluorescent molecules.
- A Polarity-Sensitive Fluorescent Amino Acid and its Incorporation into Peptides for the Ratiometric Detection of Biomolecular Interactions,
Hidetomo Yokoo, Hiroyuki Kagechika, Ayumi Ohsaki, Tomoya Hirano,
ChemPlusChem 2019.
https://doi.org/10.1002/cplu.201900489