The enzymatic synthesis of hydrocarbons from natural products is rare. However, in recent years several enzymes have been identified that convert fatty acids (particularly linear fatty acids) into terminal olefins. This process could be useful for the production of biofuels. The 1-undecene biosynthesis enzyme UndA is one example of this type of enzyme. However, it is still unclear whether its active site is a mononuclear or binuclear iron system. Finding out what the most likely active site in UndA is could allow researchers to engineer this biological system and optimize its product distributions.
To answer this question, Sam P. de Visser, University of Manchester, UK, and colleagues have devised a series of computational models and calculated the reaction mechanisms of both mononuclear and binuclear iron systems with fatty acids in the presence of dioxygen. The team used density functional theory (DFT) calculations on model systems with different protonation states of the amino acid residues around the enzyme’s active site. Pentanoic acid was used as a model substrate.
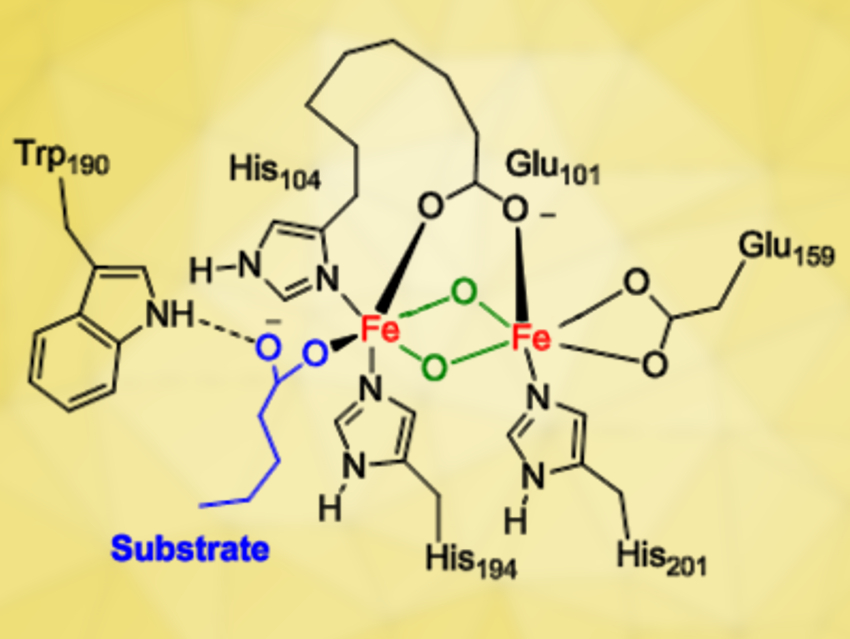
The work shows that a mononuclear iron(III)‐superoxo species (pictured below on the left) is a sluggish oxidant for decarboxylation reactions, while a binuclear iron(IV)-peroxo structure (pictured below on the right) is much more reactive. These results imply that a much more powerful oxidant is formed when dioxygen bridges two iron atoms than when it sticks to one and that a diiron structure is the more likely active site in UndA.
.jpg)
- Can a Mononuclear Iron(III)‐Superoxo Active Site Catalyze the Decarboxylation of Dodecanoic Acid in UndA to Produce Biofuels?,
Yen‐Ting Lin, Agnieszka Stańczak, Yulian Manchev, Grit D. Straganz, Sam P. Visser,
Chem. Eur. J. 2019.
https://doi.org/10.1002/chem.201903783