Alkaloids containing saturated six-membered nitrogen heterocycles such as indolizidines and quinolizidines are biologically interesting natural products and pharmaceuticals and important targets in organic synthesis. Despite synthetic efforts and impressive advances, the synthesis of functionalized indolizidine, quinolizidine, and a wide array of other azabicyclicsubunits remains a challenge.
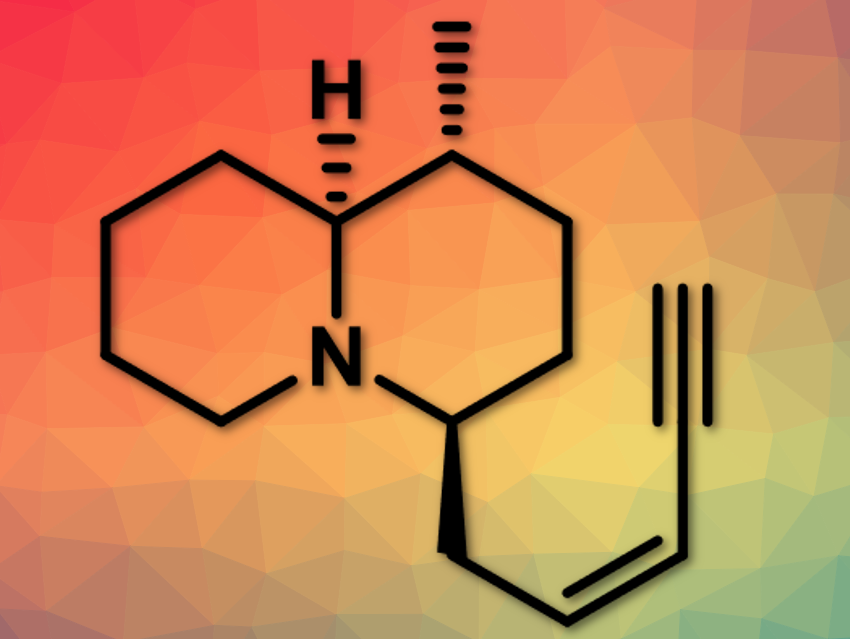
Kiyoun Lee, Catholic University of Korea, Bucheon-si, Republic of Korea, and colleagues have reported the stereoselective synthesis of quinolizidine (–)-217A. Quinolizidine(–)-217A (pictured above) is a 1,4-disubstituted quinolizidine alkaloid originally isolated from skin extracts of the Madagascan frog, Mantella baroni.
The team uses a combination of a dithiane coupling reaction and an organocatalytic aza‐conjugate addition reaction. The organocatalytic aza-conjugate addition reaction permits rapid and efficient access to the 3-methyl-2,6-cis-piperidine ring system in a diastereoselective manner. In this, 1,3-dithiane serves as a key substituent, using the gem-disubstituent effect. 2,3-trans-2,6-cis-piperidine (pictured below) is further reacted to quinolizidine (–)-217A.
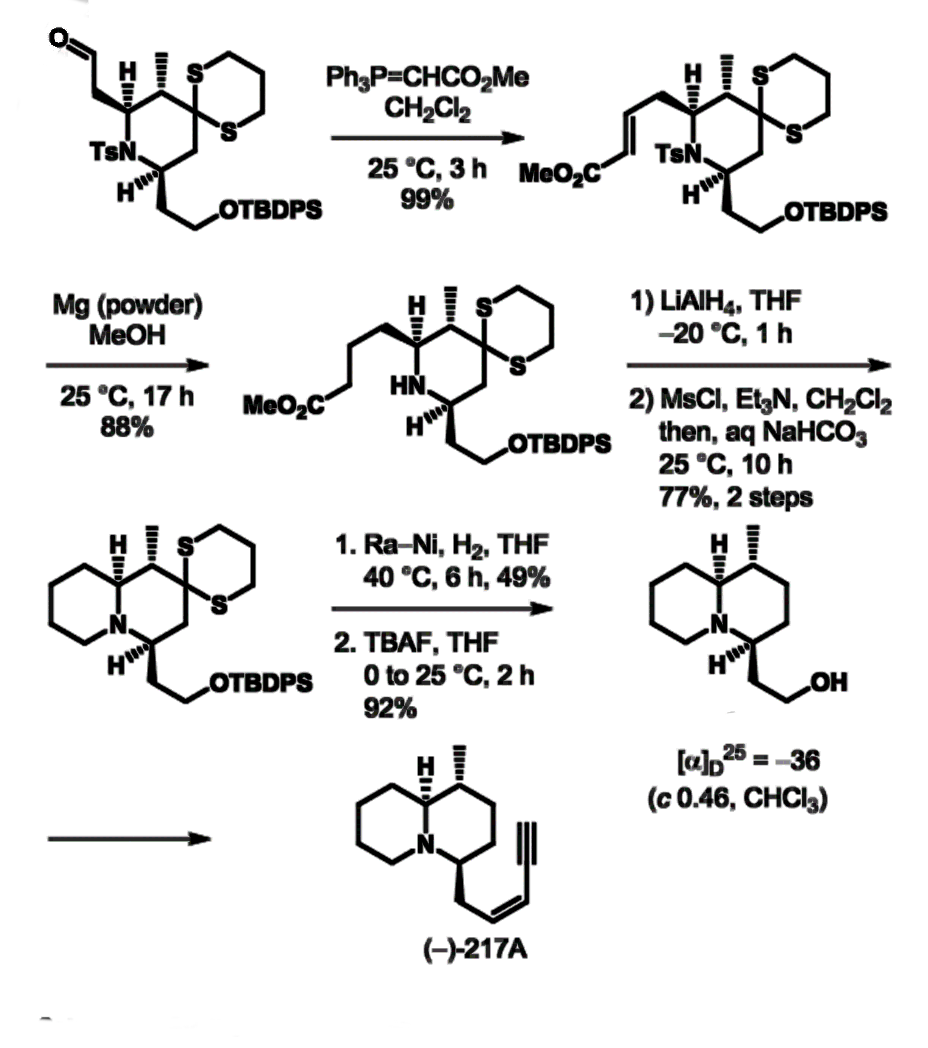
- A stereoselective formal synthesis of quinolizidine (–)‐217A,
Hosam Choi, Jiyong Hong, Kiyoun Lee,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.201901680