Pentamethylcyclopentadienyl (Cp*)CoIII catalysts have attracted much attention as an attractive alternative to noble transition metals, e.g., Cp*RhIII, for C−H activation reactions. However, the fundamental organometallic understanding of these systems is still limited.
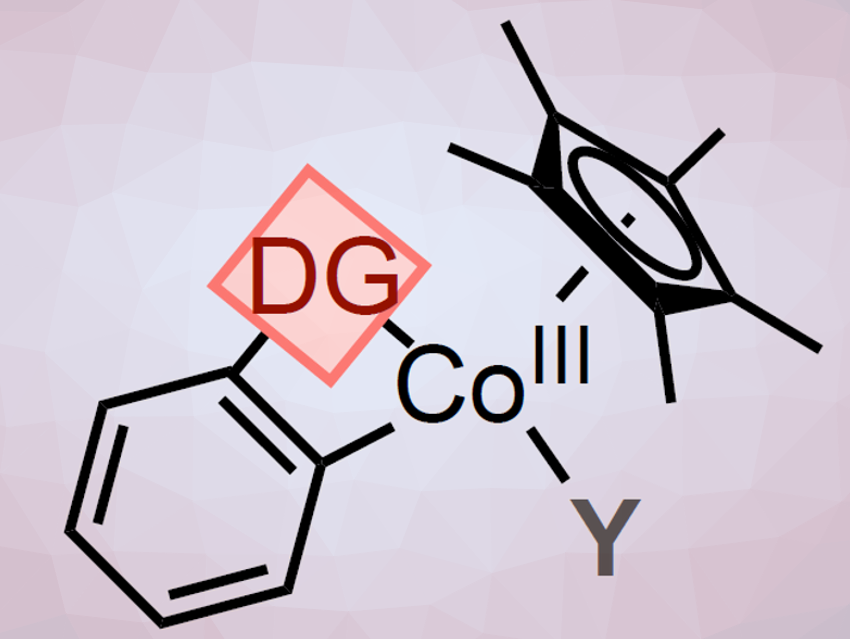
The C–H functionalization reactions are directed by weakly-coordinated functional groups, so-called directing groups (DG). The DGs contain common moieties such as ketones, aldehydes, amides, or esters. Therefore, no extra synthetic steps for their installation or removal are necessary. Usually, the weaker coordination of these DGs to the metal center leads to highly reactive metallacyclic intermediates. Their low thermodynamic stability makes their detection and/or isolation a challenge. This is especially true for first-row metals systems, such as cobalt-based platforms.
To better understand Cp*CoIII-catalyzed C–H functionalization reactions, Mónica H. Pérez-Temprano and colleagues, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, have synthesized metallacyclic Cp*CoIII complexes (A; pictured above) containing weakly-chelating DGs. The team used an oxidative addition where 2-iodo- or 2-bromobenzaldehyde was treated with [Cp*CoI(VTMS)2] in THF at room temperature (pictured below, reaction (i)). The halide R allows to synthesize, isolate, and characterize the otherwise highly reactive species.
The team found that substances with a range of DGs, such as ketones, esters, and amides, can be used. Various A-type compounds were isolated and characterized, including the first six-membered Cp*CoIII cobaltacycle with DG = acetanilide and R = I.
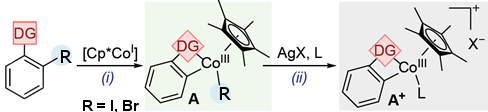
Then A was used to form direct analogs of long-sought-after cationic transient cobalt species (A+; pictured above; reaction (ii)). These cations have been widely proposed as key reactive intermediates. The researchers use coordinating ligands (L) such as THF-d8 to synthesize, isolate, and characterize these otherwise highly reactive cationic species.
Then the researchers used compounds A in C–H functionalization reactions. They found that the cyclometalated complexes are suitable catalysts for representative C–C coupling reactions, supporting the feasibility of the corresponding cationic intermediates in these transformations.
Moreover, the team found that the Cp*Co species promote cross-coupling reactions of aryl halides. The team reacted 2-iodobenzamide and diphenylacetylene as a representative system. Preliminary results provide experimental evidence that A (DG = CONH2, L = I) is a competent catalyst in the annulation reaction.
According to the researchers, their work opens new avenues for accessing previously inaccessible reactive intermediates in Cp*Co catalysis and designing novel catalytic systems that could substitute more expensive and scarce alternatives like Rh or Ir.
- Weakly Coordinated Cobaltacycles: Trapping Catalytically Competent Intermediates in Cp*CoIII Catalysis,
Sara Martínez de Salinas, Jesús Sanjosé-Orduna, Carlota Odena, Sergio Barranco, Jordi Benet-Buchholz, and Monica Helvia Perez-Temprano,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201916387