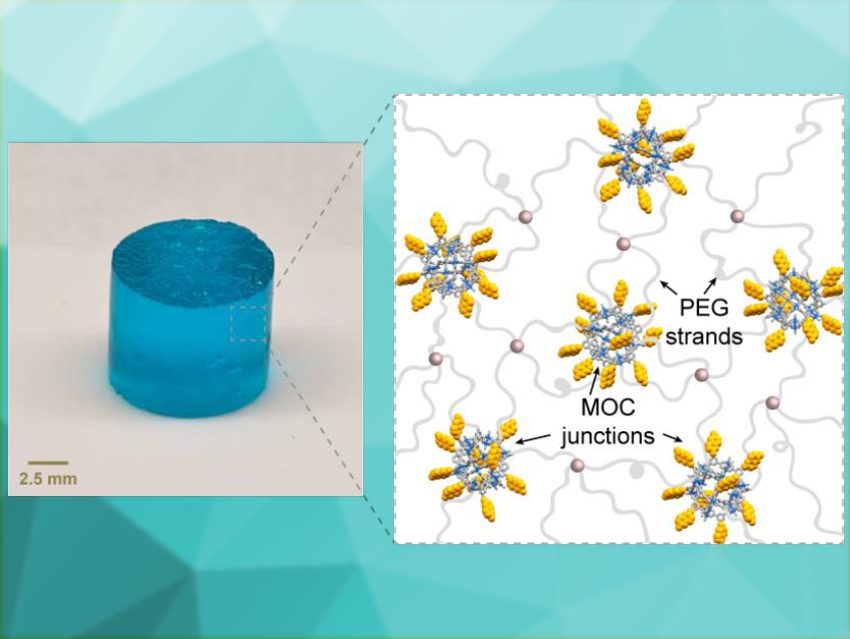
Stimuli-responsive materials can change their properties when they are exposed to an external influence, such as light, heat, pH changes, or electricity. This can be used to achieve various functions. However, the materials often return to their initial state when the stimulus is removed.
Jeremiah A. Johnson, Massachusetts Institute of Technology (MIT), Cambridge, USA, and colleagues have developed a material that switches reversibly between three sets of material and chemical properties. The team prepared a gel composed of the polymer poly(ethylene glycol) (PEG) and coumarin-functionalized benzenedicarboxylate. Upon a reaction with copper acetate, copper-containing metal–organic cages (MOCs) of the type Cu24L24 form, with coumarin as the ligand L. These MOCs link the polymer in the resulting gel (pictured above).
The material switches to different mechanical and chemical properties when exposed to UV light (pictured below). Three different copper oxidation states can be accessed—Cu(II), Cu(I), and Cu(0). Each gives a unique set of mechanical and chemical properties to the material. The Cu(0) and Cu(I) states lead to disassembly of the gel network. The Cu(I) fluid state could be used to catalyze reactions. In the Cu(0) state, the metal forms nanoparticles.
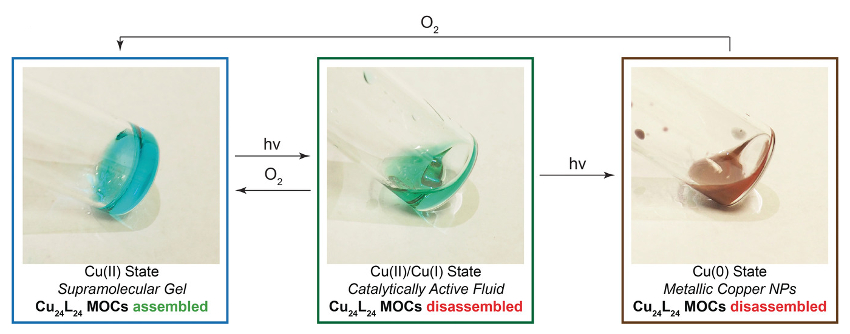
When exposed to ambient air, the material can return from the Cu(I) or Cu(0) state back to Cu(II). Since the forward photoreduction and reverse oxidation stimuli are orthogonal, the gel can switch states and it will not switch back until the reverse stimulus is applied.
- Photoswitchable Sol-Gel Transitions and Catalysis Mediated by Polymer Networks with Coumarin-Decorated Cu24L24 Metal-Organic Cages as Junctions,
Nathan J. Oldenhuis, K. Peter Qin, Shu Wang, Hong-Zhou Ye, Eric A. Alt, Adam P. Willard, Troy Van Voorhis, Stephen L. Craig, Jeremiah A. Johnson,
Angew. Chem. Int. Ed.2020.
https://doi.org/10.1002/anie.201913297