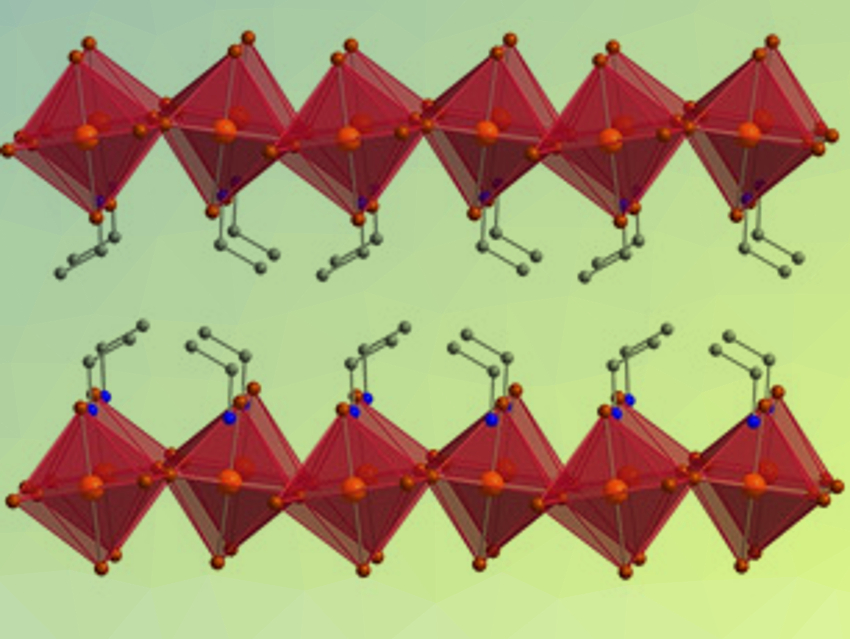
Organic–inorganic halide perovskites are intriguing materials. They combine the “softness” of organic molecules and the crystallinity of inorganic solids. Squeezing halide perovskites at high pressures can dramatically change their properties. Copper chloride perovskites, for example, are conductive, but only at very high pressures that are difficult to achieve (> 50 GPa).
Hemamala I. Karunadasa, Stanford University and SLAC National Accelerator Laboratory, Menlo Park, both CA, USA, and colleagues have found that the layered copper bromide perovskite (EA)2CuBr4 (pictured, EA = ethylammonium) is conductive at much lower pressures (< 3 GPa). The team crystallized the perovskite from solution and pressed samples in diamond‐anvil cells. By measuring changes in structure, optical properties, and electronic transport in these samples, they determined how pressure-induced structural changes cause the changed electronic behavior. The researchers attribute the sudden onset of electronic conductivity to a phase transition that allows singly occupied orbitals to overlap and, thus, provides a conduction pathway.
The greatly improved pressure response in copper bromide perovskites may allow their use in technology. The researchers speculate that these reversible pressure-induced changes in perovskite films could, for example, be used for printing transient electronic circuits or for registering impact or strain.
- High Compression-Induced Conductivity in a Layered Cu-Br Perovskite,
Adam Jaffe, Stephanie A. Mack, Yu Lin, Wendy L. Mao, Jeffrey B. Neaton, Hemamala I. Karunadasa,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201912575