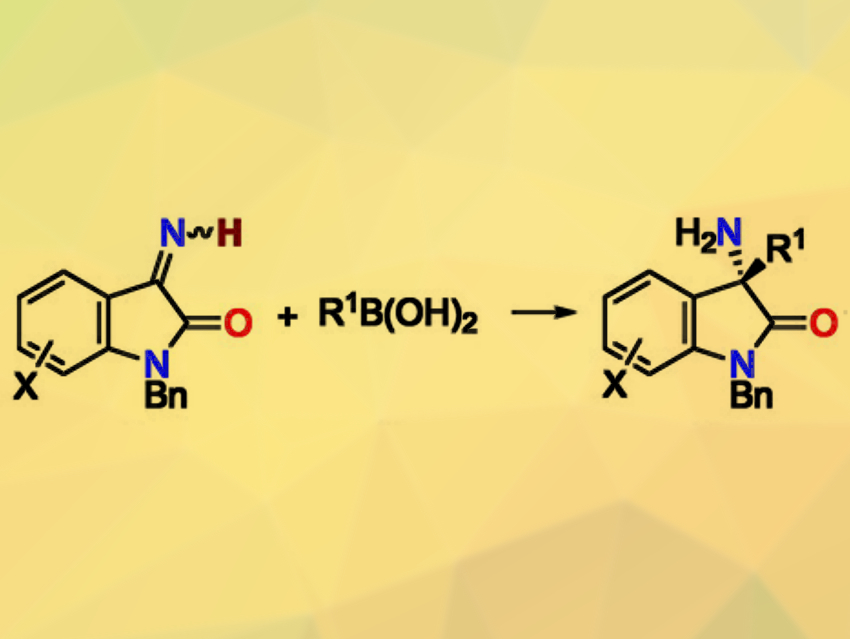
The catalytic enantioselective addition of boronic acids to ketimines is useful for synthesizing enantioenriched amines next to tetrasubstituted carbon stereocenters. This reaction has been well-developed for N-substituted ketimines. However, it remains challenging for N-unsubstituted ketimines. Existing approaches for this reaction require preformed boroxines as a boronic-acid equivalent and stoichiometric amounts of a base.
Hiroyuki Morimoto, Takashi Ohshima, Kyushu University, Fukuoka, Japan, Tamio Hayashi, Nanyang Technological University, Singapore, and colleagues have developed a rhodium(I)/chiral-diene-catalyzed enantioselective addition of boronic acids to N-unsubstituted isatin-derived ketimines (pictured). The team used a chiral diene ligand with a secondary amide moiety, catalytic amounts of K2CO3 as a base, and various boronic acids as reactants. They obtained the desired products in high yields and enantioselectivities.
A combination of experimental and computational mechanistic studies revealed that a hydrogen-bonding interaction between the amide moiety of the diene ligand and the N-unsubstituted ketimines is crucial for stabilizing the transition state. This effect leads to high reactivity and enantioselectivity.
- Rhodium(I)/Chiral Diene-Catalyzed Enantioselective Addition of Boronic Acids to N-Unsubstituted Isatin-Derived Ketimines,
Ryohei Yonesaki, Ibuki Kusagawa, Hiroyuki Morimoto, Tamio Hayashi, Takashi Ohshima,
Chem. Asian J. 2020.
https://doi.org/10.1002/asia.201901745