Iron oxides and hydroxides are abundant in nature. Atomically precise molecular metal-oxo clusters provide ideal models to understand metal oxide surfaces and form-function relationships. However, preparing and isolating these compounds remains challenging.
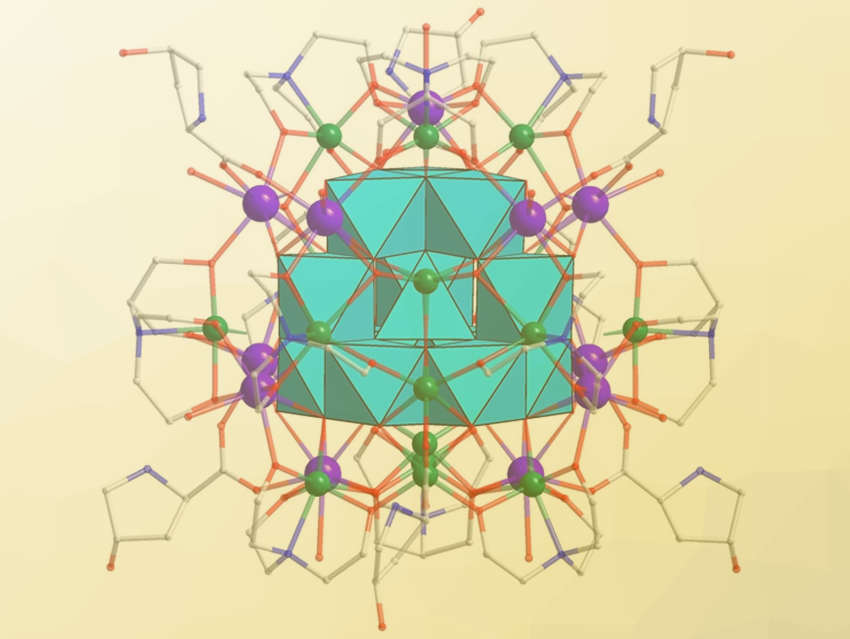
La-Sheng Long, Xiang-Jian Kong, Xiamen University, China, May Nyman, Oregon State University, Corvallis, USA, and colleagues have synthesized iron-oxo clusters (example pictured) stabilized by rare earths (Y and Gd) along with the amino acid ligands trans-4-hydroxyl-L-proline (Hyp) and triethanolamine (TEOA-H3). The clusters were prepared in solution from Y(ClO4)3, Gd(ClO4)3, Fe(ClO4)3 • 9 H2O, and the amino ligands.
The team prepared four isostructural compounds, containing 29 or 32 iron centers in the core and 12 or 16 rare-earths in the shell. The products are the largest lanthanide-iron-oxo clusters to date. They all feature the ϵ‐{Fe13} Keggin cluster in their core (pictured in turquoise). Surrounding the inner Fe13 core are the outer metal ions (Y or Gd, pictured in purple; Fe, pictured in green).
The clusters are stable upon dissolution in both water and organic solvents. The compositional flexibility suggests that it could be possible to prepare an entire family of compounds. According to the researchers, this could provide opportunities to construct molecular forms of important magnetic materials.
- Atomically Precise Lanthanide‐Iron‐Oxo Clusters Featuring the ϵ‐Keggin Ion,
Xiu‐Ying Zheng, Ming‐Hao Du, Mehran Amiri, May Nyman, Qiang Liu, Tao Liu, Xiang‐Jian Kong, La‐Sheng Long, Lan‐Sun Zheng,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201904636