Lithium–air (Li–O2) batteries have a high theoretical specific energy. However, high overpotentials and issues with side reactions can prevent their practical use. Suitable catalysts could reduce these overpotentials. Redox mediators (RMs), for example, could be promising catalysts for high-performance Li-O2 batteries.
Some natural enzymes have been used as RMs. Heme groups, for example, can take effect in both discharge and charge processes because of the suitable redox potential of the Fe3+/Fe2+ redox pair. However, natural enzymes can have insufficient stability during battery cycling. Artificial “nanozymes”, i.e., nanomaterials with enzyme‐like catalytic activities, could be used as stable alternatives.
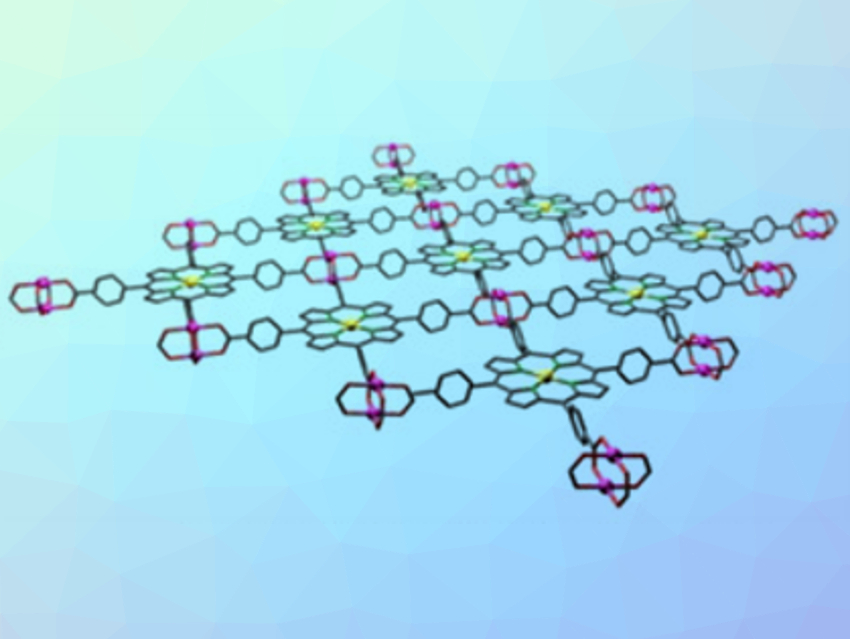
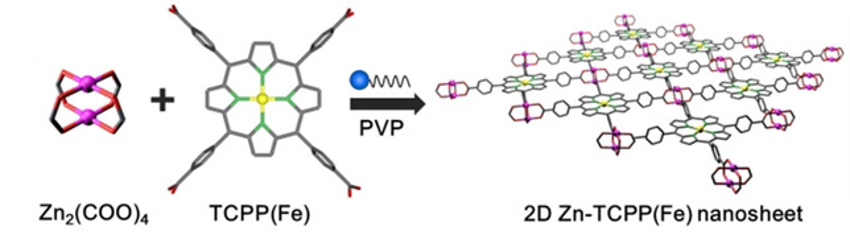
Ping He, Nanjing University, China, Haoshen Zhou, Nanjing University and National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, and colleagues have developed Zn-TCPP(Fe) nanozymes (pictured) for this application. These 2D nanosheets are composed of Fe(III) tetra(4-carboxyphenyl)porphine (TCPP(Fe)) linkers and zinc-based paddlewheel-type nodes. The team synthesized the Zn-TCPP(Fe) nanozymes using a surfactant-assisted method, with polyvinylpyrrolidone (PVP) as the surfactant.
The Zn-TCPP(Fe) nanozymes can act as bifunctional RMs for both discharge and charge processes in lithium–air batteries. They coordinate superoxide intermediates and act as molecular shuttles of superoxide species and electrons. As a consequence, Li-O2 batteries with these nanozymes have improved discharge capacities and superior cycling stabilities compared to those without Zn-TCPP(Fe) additives.
- Using a Heme-Based Nanozyme as Bifunctional Redox Mediator for Li–O2 Batteries,
Xiaowei Mu, Yufeng Liu, Xueping Zhang, Hui Wei, Ping He, Haoshen Zhou,
Batteries Supercaps 2020.
https://doi.org/10.1002/batt.201900196