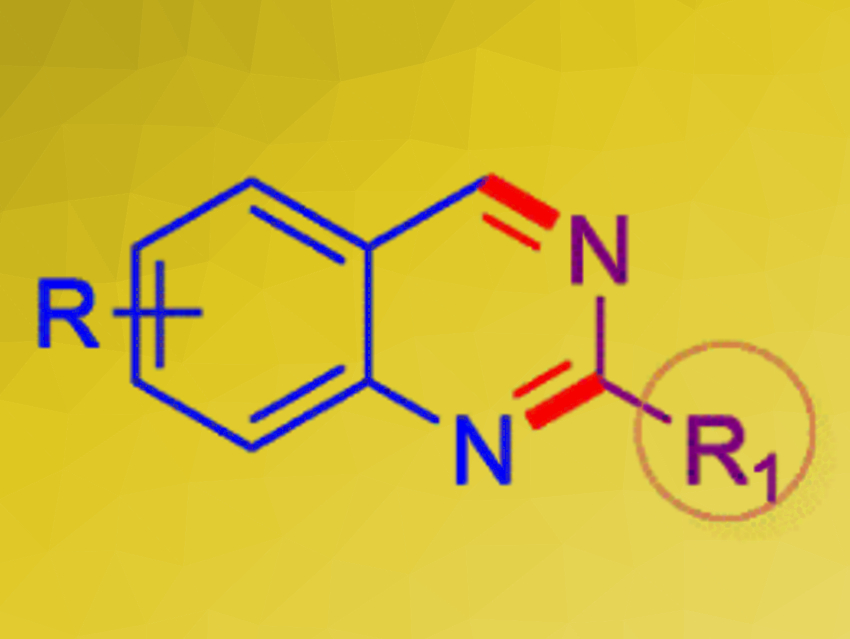
Quinazolines (pictured) are aromatic N-heterocycles. They are found in several important pharmaceutically active compounds, e.g., with anticancer, antibacterial, and antiviral activities. There is a range of synthetic approaches to quinazolines that use metal catalysts. Organocatalytic methods for the preparation of substituted quinazolines are much rarer in comparison.
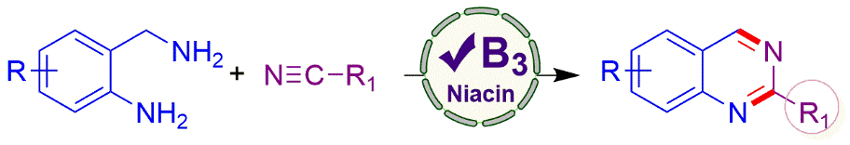
Chandi C. Malakar, National Institute of Technology Manipur, Imphal, India, and colleagues have developed an organocatalytic protocol for the synthesis of diversely substituted quinazolines promoted by vitamin B3 (niacin), using nitriles as a CN source. The team used various 2-aminobenzylamines and nitriles as substrates, niacin as the catalyst, and dimethylsulfoxide (DMSO) as the solvent. The reaction was performed at 110 °C under air.
The desired substituted quinazolines were obtained in good yields. The approach provides good functional group tolerance. The researchers propose a reaction mechanism in which catalytic amounts of hydrogen peroxide are generated by oxidation in air, which can react with niacin and oxygen to form a cationic N-oxide radical. This radical can abstract a hydrogen atom from the amine reactant, leading to an imine intermediate. This intermediate undergoes an addition to the nitrile, ring closing, and a dehydroamination to give the product.
- Niacin as a Potent Organocatalyst towards the Synthesis of Quinazolines using Nitriles as C-N Source,
Raghuram Gujjarappa, Nagaraju Vodnala, Velma Ganga Reddy, Chandi C Malakar,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.201901651