Flexible zinc–air batteries (ZAB) are promising battery candidates for flexible electronic devices because they are safe and have a high energy density. However, they contain water-based electrolytes, which freeze at low temperatures. The reactions in zinc-air batteries that involve oxygen also become much slower when the temperature decreases.
Yuan Chen, University of Sydney, Australia, and colleagues have boosted the performance of zinc-air batteries at low temperatures using two approaches. They developed a catalyst with much higher activity for the oxygen reactions to minimize the activity loss at low temperatures, and they synthesized an anti-freeze hydrogel to be used as the electrolyte.
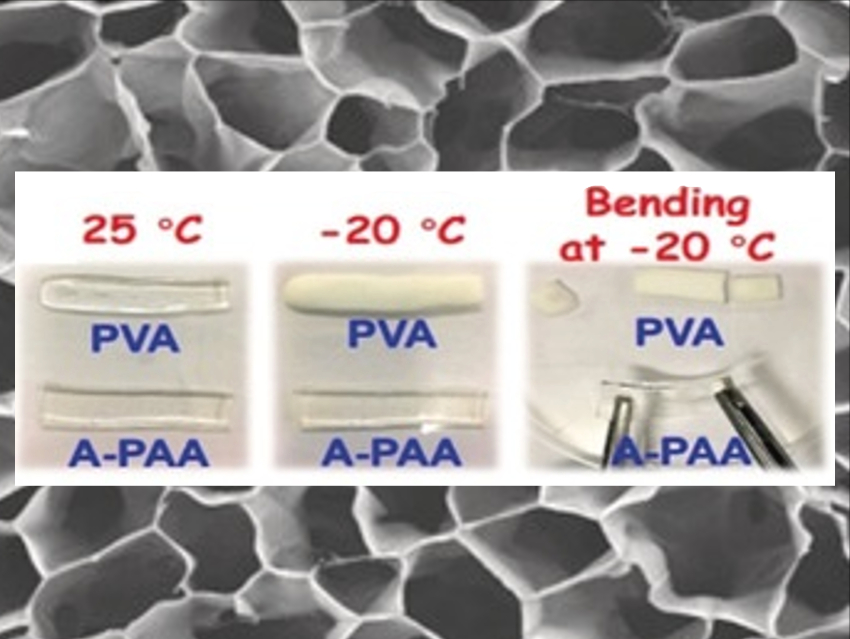
The catalyst is a 1D fibrous electrocatalyst with a bamboo-like structure. The team prepared the catalyst using a co‐axial electrospinning method with both high‐molecular‐weight polyacrylonitrile (PAN) and low‐weight polyvinylpyrrolidone (PVP). Iron and cobalt salts were added to the PAN solution to create active catalytic sites. The team also used a hydrogel electrolyte with polarized terminal groups, i.e., carboxylate groups in alkalified polyacrylic acid (pictured, denoted as A‐PAA). This enhances the interaction between the hydrogel and water significantly and prevents the formation of ice crystals.
The catalyst shows one of the lowest reversible overpotentials for the oxygen reduction/evolution reactions (ORR/OER) reported to date. The freezing temperature of the hydrogel electrolyte can drop to –36 °C, and the assembled zinc-air battery works well at –20 °C with excellent mechanical flexibility. This could allow applications in various wearable devices.
- A Flexible Rechargeable Zinc-Air Battery with Excellent Low-Temperature Adaptability,
Zengxia Pei, Ziwen Yuan, Chaojun Wang, Shenlong Zhao, Jingyuan Fei, Li Wei, Junsheng Chen, Cheng Wang, Rongjie Qi, Zongwen Liu, Yuan Chen,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201915836