Difluoromethyl (CF2H) groups are commonly used in pharmaceutical chemistry. In contrast to the extensively studied trifluoromethyl (CF3) group, research on difluoromethyl chemistry is lagging behind somewhat. The volatile difluorodiazoethane (CF2HCHN2), for example, has been used to introduce difluoromethyl groups to diverse molecular frameworks. However, it is very reactive and difficult to handle.
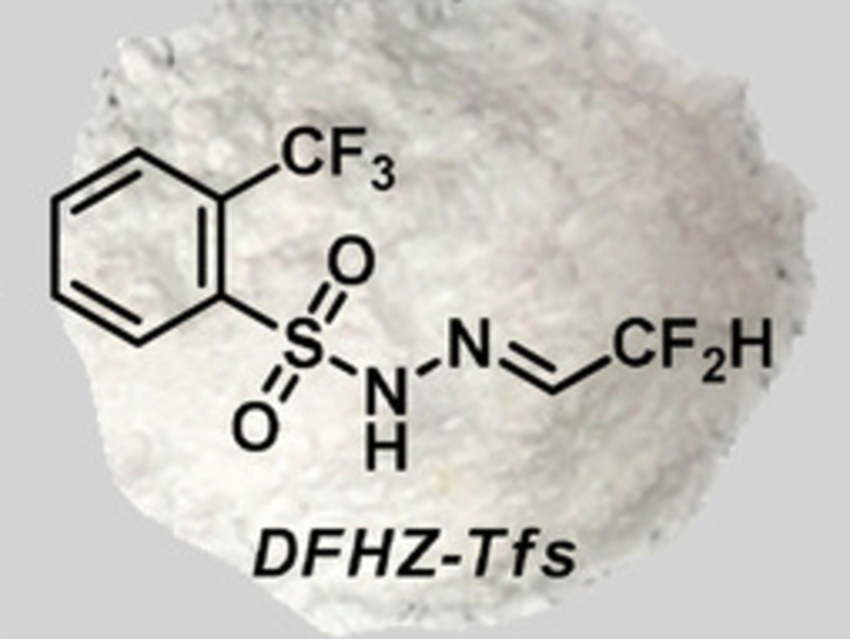
Xihe Bi, Northeast Normal University, Changchun, China, and Nankai University, Tianjin, China, and colleagues have found that the bench-stable difluoroacetaldehyde N-triftosylhydrazone (DFHZ-Tfs, pictured above) can be used as an operationally safe diazo precursor for the in-situ generation of reactive diazo species. DFHZ-Tfs was prepared from commercially available ethyl difluoroacetate in two steps: a reduction with LiAlH4 and a condensation with o-trifluoromethylbenzenesulfonyl hydrazide.
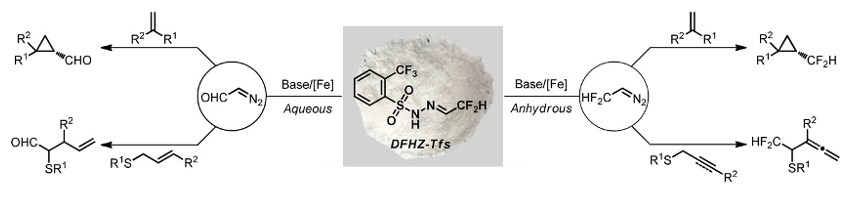
Using DFHZ-Tfs, CF2HCHN2 can be generated under anhydrous reaction conditions and diazoacetaldehyde (CHOCHN2) can be created under aqueous conditions (pictured below). From these reactive intermediates, CF2H (or CHO)-containing products were obtained in good to excellent yields, with a broad substrate scope and excellent functional group tolerance. Due to its stability and operational simplicity, as well as the mild and controlled decomposition conditions, DFHZ-Tfs could have a wide range of applications in organic synthesis.
- Difluoroacetaldehyde N-Triftosylhydrazone (DFHZ-Tfs) as a Bench-Stable Crystalline Diazo Surrogate for Diazoacetaldehyde and Difluorodiazoethane,
Yongquan Ning, Xinyu Zhang, Yi Gai, Yuanqing Dong, Paramasivam Sivaguru, Yingying Wang, Bhoomireddy Rajendra Prasad Reddy, Giuseppe Zanoni, Xihe Bi,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202000119