Highly fluorinated benzenes (HFB) are useful, but underused, solvents and ligands in organic synthesis and catalysis. The interplay between a reactive metal center and a coordinating solvent can be used to tune the reactivity in catalytic systems. Cationic rhodium diphosphines, for example, are pre-catalysts for a variety of important transformations. However, with very weakly binding substrates, the initial interaction with the metal center can be disrupted by a more strongly binding solvent. Thus, it could be possible to develop new, more active catalysts that incorporate the very weakly binding HFB ligands.
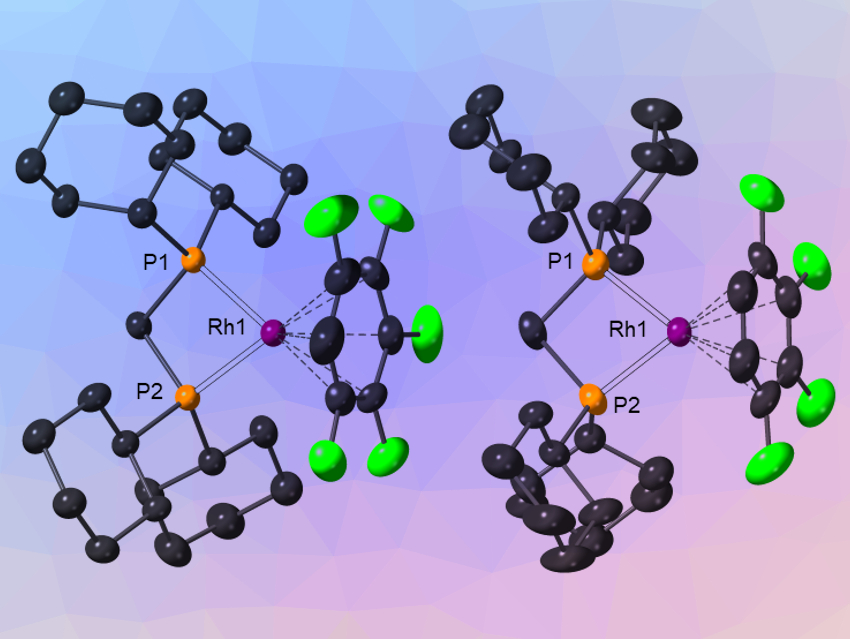
Andrew S. Weller, University of Oxford and University of York, both UK, and colleagues have investigated how cationic rhodium fragments interact with HFBs. 1,2,3‐F3C6H3, 1,2,3,4‐F4C6H2, and 1,2,3,4,5‐F5C6H were found to bind with cationic [Rh(Cy2P(CH2)xPCy2)]+ fragments (x = 1,2; Cy = cyclohexyl, example pictured). The team used the non‐coordinating [Al{OC(CF3)3}4]− anion as a partner to allow the synthesis of these complexes. They were prepared from precursors with norbornadiene ligands by hydrogenation.
The team found that the complexes are useful in catalysis—even with weakly binding substrates—due to the weakly binding HFB ligand. However, this requires that they are used in conjunction with an HFB solvent to avoid interference by the solvent molecules. The team also demonstrated that the HFB ligands can be readily substituted by other weakly binding ligands such as isobutene, opening up new opportunities in synthesis.
- Synthesis of Highly Fluorinated Arene Complexes of [Rh(Chelating Phosphine)]+ Cations, and their use in Synthesis and Catalysis,
Alasdair I. McKay, James Barwick‐Silk, Max Savage, Michael C. Willis, Andrew S. Weller,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201904668