Halide perovskite nanocrystals have shown promise in high-performing solar cells and light-emitting diodes (LEDs). Incorporating thallium into these materials could provide additional bandgap tunability. One example of this effect is Cs2AgTlBr6, which has a very low bandgap of 0.95 eV. Thallium halide nanocrystals could also prove useful for radiation detectors and near-infrared (NIR) optical components. However, there are only a few examples of nanocrystal syntheses involving thallium.
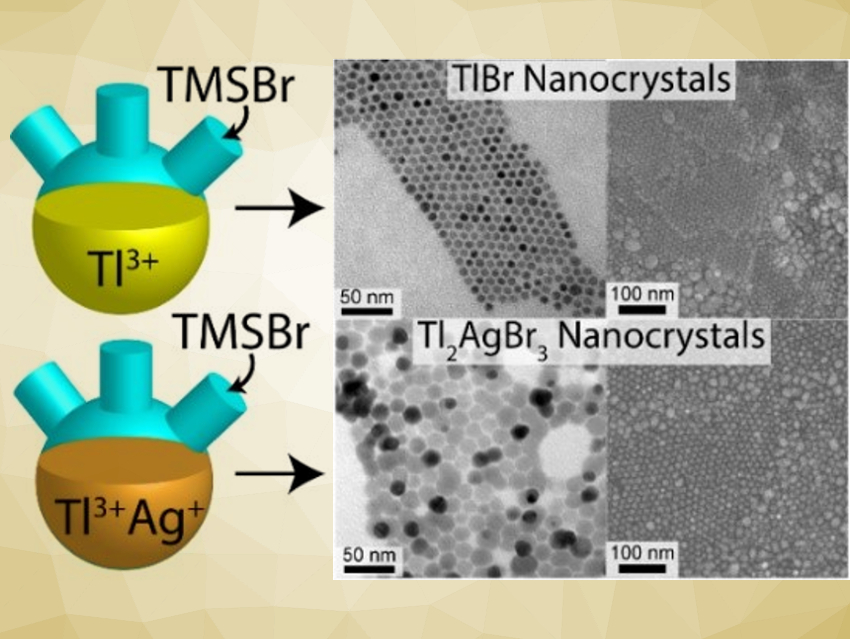
Brian A. Korgel and colleagues, The University of Texas at Austin, USA, have developed colloidal syntheses of uniform TlBr and Tl2AgBr3 nanocrystals. The TlBr synthesis involves the injection of trimethylsilyl bromide (TMSBr) into heated solutions of thallium(III) or thallium(I) acetate in oleylamine, oleic acid, and octadecene. Tl2AgBr3 nanocrystals were obtained using an analogous reaction with additional silver(I) acetate.
The team found that the TlBr nanocrystals made with thallium(III) were uniform enough to self-assemble into face-centered cubic (FCC) superlattices. The band gaps of the TlBr and Tl2AgBr3 nanocrystals are both 3.1 eV, which is too large for most solar-cell and LED applications; however, the ability to deposit thin films on large-area surfaces might allow a new class of radiation scintillator materials to be developed.
- Synthesis of TlBr and Tl2AgBr3 Nanocrystals,
Timothy Siegler, Tushti Shah, Daniel Houck, Mokshin Suri, Yangning Zhang, Brian A. Korgel,
ChemNanoMat 2020.
https://doi.org/10.1002/cnma.202000100