In chiral Brønsted-base catalysis, the basicity of the catalyst can restrict the scope of pronucleophiles to highly acidic compounds. Pronucleophiles are compounds that can be converted into nucleophiles by deprotonation. This issue limits the range of possible enantioselective transformations when using conventional Brønsted-base catalysts.
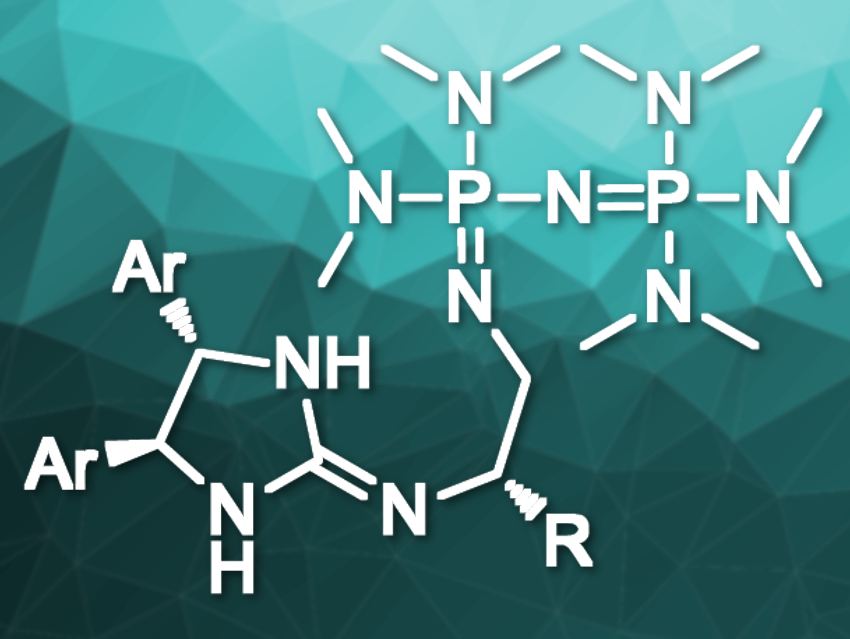
Masahiro Terada, Tohoku University, Sendai, Japan, and colleagues have developed chiral strong Brønsted-base catalysts containing two different organobase functionalities (pictured). The team’s molecular design provides dual functionality: it contains both a P2-phosphazene organosuperbase and a chiral guanidine that can act as a hydrogen-bond donor for substrate recognition. Organosuperbases are organic compounds with high basicities and low nucleophilicities.
The catalyst was prepared via a convergent synthesis from three readily accessible components: a triaminophosphonium salt as the P2‐phosphazene precursor, a chiral cyclic thiourea as the guanidine precursor, and a chiral 1,2‐diaminoethane derivative as the linker. This approach makes it easy to optimize the catalyst by changing its components.
The researchers demonstrated the reactivity of the chiral catalyst with an unprecedented enantioselective direct Mannich-type reaction of a pronucleophile with relatively low acidity, α‐phenylthioacetate. The reaction produces an enantioenriched α-thio-β-aminocarbonyl scaffold, which is a synthetically useful building block. According to the researchers, the approach could broaden the scope of chiral Brønsted-base catalysis in organic synthesis.
- Development of Chiral Organosuperbase Catalysts Consisting of Two Different Organobase Functionalities,
Azusa Kondoh, Masafumi Oishi, Hikaru Tezuka, Masahiro Terada,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202001419