Graphene-like structures are popular in materials research due to their promising, often unusual properties. It is known that structural defects can promote changes in these properties. However, the specific effects of different types of defects are not entirely clear yet.
Araceli G. Campaña, University of Granada, Spain, Ermelinda Maçôas, University of Lisbon, Portugal, and colleagues have investigated the influence of heptagonal rings on the optoelectronic properties of nanographenes. The team synthesized a family of well-defined nanographene ribbons that consist of three fused hexa-peri-hexabenzocoronene (HBC)-type units each.
Two of the structures have a saddle-shaped HBC central unit (pictured below on the left), whose curvature originates from a heptagonal carbocycle. These compounds were synthesized starting from a heptagon‐containing HBC bearing two aryl bromides via a double Sonogashira coupling, a double Diels–Alder reaction, and a Scholl‐type cyclodehydrogenation reaction.
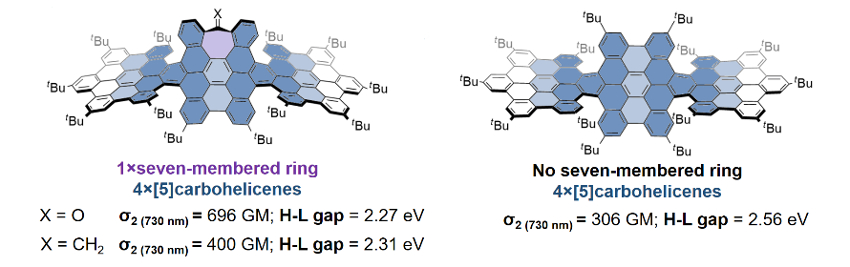
The presence of a carbonyl-substituted heptagonal ring (tropone ring, pictured in violet below, X = O) improves the non-linear optical properties of the ribbons, increasing the efficiency of upconverted emission by two-photon excitation compared with a reference compound without the seven-membered ring (see the two-photon cross-sections σ2 listed below, 1 GM = 10−50 cm4 s photon–1). The seven-membered ring also reduces the HOMO-LUMO gap (HOMO = highest occupied molecular orbital, LUMO = lowest unoccupied molecular orbital). This work shows that structural defects can be harnessed for tuning the optical response of nanographenes and designing specific structures for particular applications.
- Two‐photon absorption enhancement by the inclusion of a tropone ring in distorted nanographene ribbons,
Silvia Castro-Fernández, Carlos M Cruz, Inês F. A. Mariz, Irene R. Márquez, Vicente G. Jiménez, Lucía Palomino-Ruiz, Juan M. Cuerva, Ermelinda Maçôas, Araceli G. Campaña,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202000105