Hydrogen peroxide, best known as a bleaching agent, can also be used, e.g., as a green oxidant. The direct synthesis of hydrogen peroxide from H2 and O2 could be an alternative to the commercially used Riedl-Pfleiderer process, which involves the cyclic auto-oxidation of anthraquinones. However, the design of efficient catalysts with high activity and H2O2 selectivity for the direct reaction is challenging.
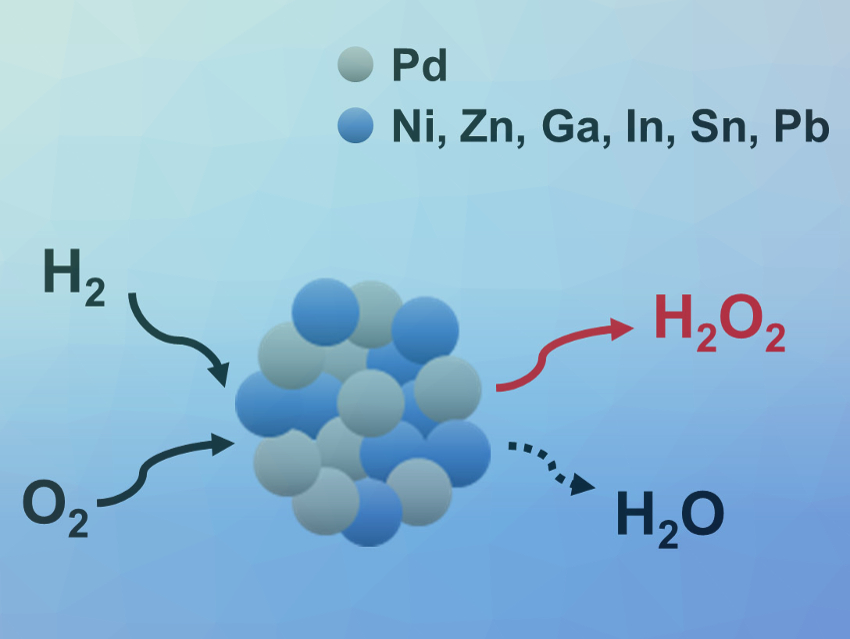
Silke Behrens, Karlsruhe Institute of Technology (KIT) and University of Heidelberg, both Germany, and colleagues have used bimetallic, Pd-based nanocrystals as catalyst precursors for the direct synthesis of H2O2. The team studied the effects of metal doping on these Pd-based catalysts. They synthesized a library of bimetallic PdM nanocrystals with tunable compositions (M = Ni, Zn, Ga, In, Sn, Pb) from solutions of the metal acetylacetonate (acac) or acetate (ac) precursors with oleylamine (OLAM) and trioctylphosphine (TOP) at 200–300 °C. The nanocrystals were immobilized on an acid-pretreated TiO2 support.
The direct H2O2 synthesis using the resulting catalysts was investigated at 30 °C and 40 bar in ethanol. The team found that tin- or gallium-doping of the palladium nanocrystals improves the catalyst performance, even in the absence of acid or halide promotors. The addition of acid promotors to the reaction medium can cause metal leaching and reactor corrosion, which makes the acid- and halide-free H2O2 synthesis with Sn- or Ga-doped Pd catalysts attractive.
- Pd-Based Bimetallic Nanocrystal Catalysts for the Direct Synthesis of H2O2,
Sheng Wang, Dmitry E. Doronkin, Martin Hähsler, Xiaohui Huang, Di Wang, Jan-Dierk Grunwaldt, Silke Behrens,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202000407