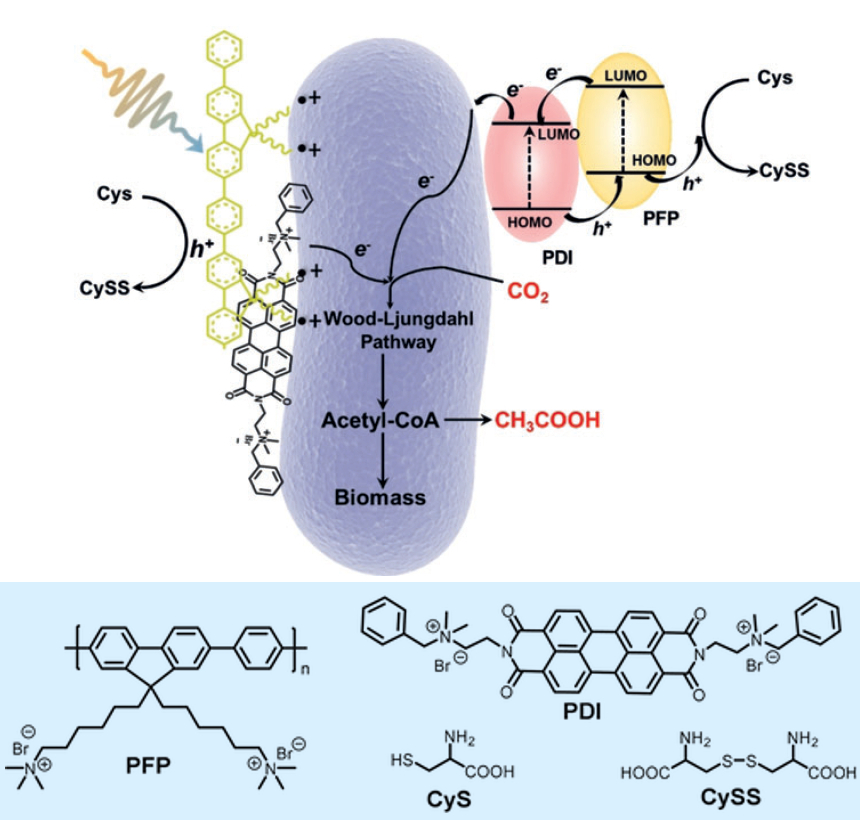
Photosynthetic biohybrid systems can take advantage of both the light-harvesting ability of semiconductors and the synthetic abilities of living cells. However, high-performance photosynthesis requires good solar energy utilization, hole/electron separation efficiency, and electron transfer between the semiconductor and biological cells, which are challenging to achieve.
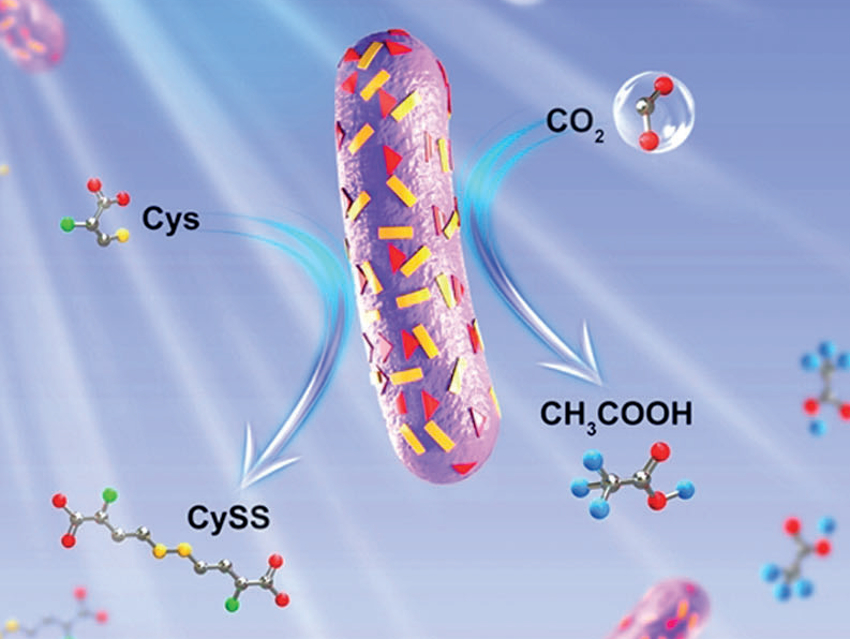
Shu Wang, Beijing National Laboratory for Molecular Sciences, Chinese Academy of Sciences, Feng Li, Qingdao Agricultural University, China, and colleagues have developed a photosynthetic system that can reduce carbon dioxide to acetic acid. It consists of organic semiconductors coated onto the surface of the non-photosynthetic bacteria Moorella thermoacetica. The organic semiconductors the team used are the electron-transporting (n-type) perylene diimide (PDI, pictured below) and the hole-transporting (p-type) poly(fluorene-co-phenylene) (PFP, pictured below). They were added to the culture medium of the bacteria to coat their surface.
The semiconductors act as photosensitizers on the bacteria surface. On exposure to light, the bacteria harvests photoexcited electrons from the PFP/PDI layer, which then drive a metabolic pathway to synthesize acetic acid from CO2. Cysteine (Cys) is used as an electron donor in this process and dimerized to CySS (pictured below). The biohybrid has a solar-to-chemical conversion efficiency of approximately 1.6 %.
- Solar-Powered Organic Semiconductor–Bacteria Biohybrids for CO2 Reduction into Acetic Acid,
Panpan Gai, Wen Yu, Hao Zhao, Ruilian Qi, Feng Li, Libing Liu, Fengting Lv, Shu Wang,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202001047