Helicenes are polycyclic aromatic compounds with chiral helical shapes. Their enantiopure forms show strong chiroptical activity and potentially useful emission properties. The light they emit is circularly polarized, i.e., the pure enantiomers emit either more right-handed or more left-handed circularly polarized light. This circularly polarized luminescence could have applications in, e.g., cryptography, organic light-emitting diodes (OLEDs), bioimaging, or photochemistry.
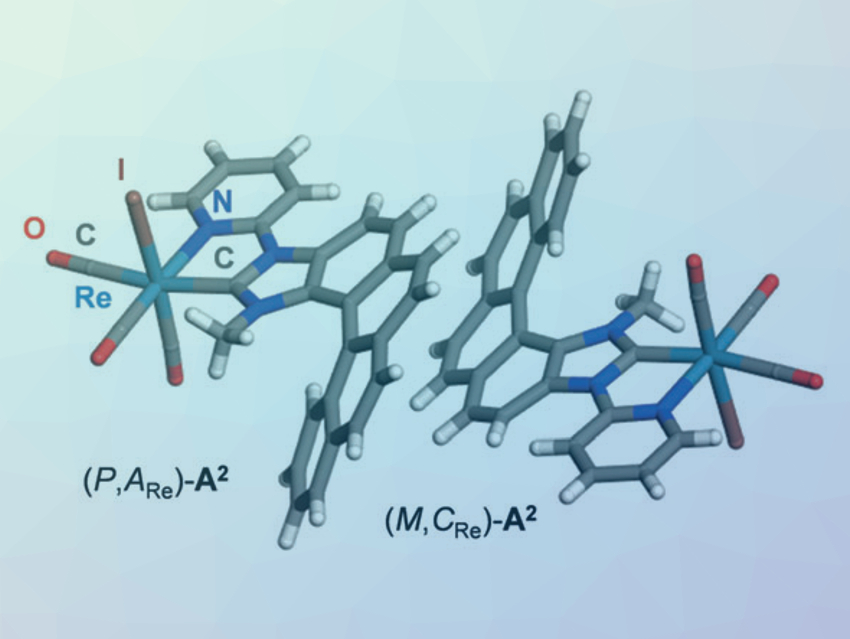
J. A. Gareth Williams, Durham University, UK, Jochen Autschbach, University at Buffalo, State University of New York, USA, Jeanne Crassous, Université de Rennes, CNRS, France, and colleagues have studied the chemistry of helicene-type N-heterocyclic carbenes (NHCs) and their complexes with rhenium. The team synthesized the first enantiopure “chiral‐at‐rhenium” complexes of the form fac‐ReX(CO)3(NHC) (example pictured, X = Cl,I), with an NHC that features a [5]helicene unit.
The researchers started from [5]helicene‐imidazole, which was coupled with 2‐bromopyridine and methylated with MeI. The resulting imidazolium iodide was reacted with ReCl(CO)5 in the presence of K2CO3 to give the iodo rhenium complex. To synthesize the chloro complex, the imidazolium iodide was first converted to the corresponding chloride salt by anion metathesis.
Pure enantiomers of the chiral Re complexes were obtained using chiral high-performance liquid chromatography (HPLC). They show intense circularly polarized phosphorescence. The team observed phosphorescence lifetimes up to 4000–6000 times longer than for other NHC-based Re complexes. Both the stereochemistry and the halogen ligands affect the photophysics of the complexes—for example, the quantum yields of the iodo complexes are an order of magnitude lower than for their chloro counterparts and the lifetimes are an order of magnitude smaller.
- Long‐lived circularly‐polarized phosphorescence in helicene‐NHC‐rhenium(I) complexes: the influence of helicene, halogen and stereochemistry on emission properties,
Jeanne Crassous, Etienne S. Gauthier, Laura Abella, Nora Hellou, Benoît Darquié, Elsa Caytan, Thierry Roisnel, Nicolas Vanthuyne, Ludovic Favereau, Monika Srebro-Hooper, J. A. Gareth Williams, Jochen Autschbach,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202002387