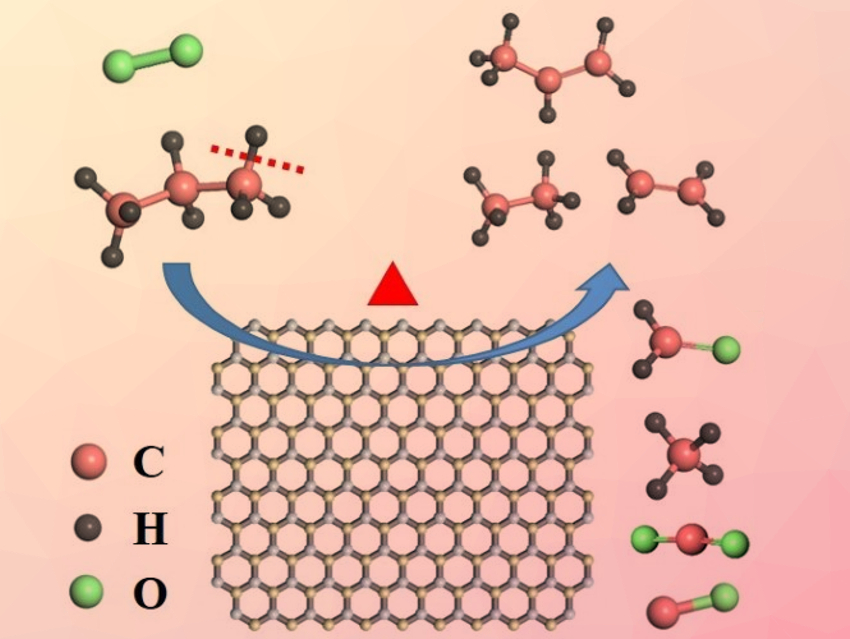
Hexagonal boron nitride (h-BN) and similar boron-based materials are useful catalysts for selectively converting light alkanes to valuable olefins. However, their mode of action is not well-understood.
Victor Fung, Zili Wu, Oak Ridge National Laboratory, TN, USA, Weixin Huang, University of Science and Technology of China, Heifei, and colleagues have investigated the reaction mechanism of alkane conversion over h-BN and supported boron oxide catalysts of the type BOx/SiO2. They used the oxidative dehydrogenation of propane (ODHP) as a model reaction. The catalyst was placed in a heated quartz tube under a gas mixture consisting of C3H8, O2, and He (C3H8:O2:He = 1:1:38). The team used synchrotron vacuum ultraviolet photoionization mass spectroscopy (SVUV‐PIMS) and density functional theory (DFT) calculation to investigate the mechanism.
The researchers observed gas-phase methyl radicals (CH3∙), which point to gas-phase radical pathways, over all tested boron-based catalysts. The results of calculations indicate that propene is mainly generated on the catalyst surface via the C–H activation of propane, while C2 and C1 products are formed via both surface-mediated and gas-phase pathways. The work, which presents a clear mechanistic picture for ODHP over boron-based materials, could be helpful for the design of more efficient catalysts for the conversion of light alkanes to olefins.
- Radical Chemistry and Reaction Mechanisms of Propane Oxidative Dehydrogenation over Hexagonal Boron Nitride Catalysts,
Xuanyu Zhang, Rui You, Zeyue Wei, Xiao Jiang, Jiuzhong Yang, Yang Pan, Peiwen Wu, Qingdong Jia, Zhenghong Bao, Lei Bai, Mingzhou Jin, Bobby Sumpter, Victor Fung, Weixin Huang, Zili Wu,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202002440