Diffusion-ordered NMR spectroscopy (DOSY) is an NMR method that can be used to investigate diffusion processes. DOSY has been used, for example, to study host-guest complexation and to measure binding affinities. However, the method has been rarely used for the kinetic characterization of supramolecular complexes.
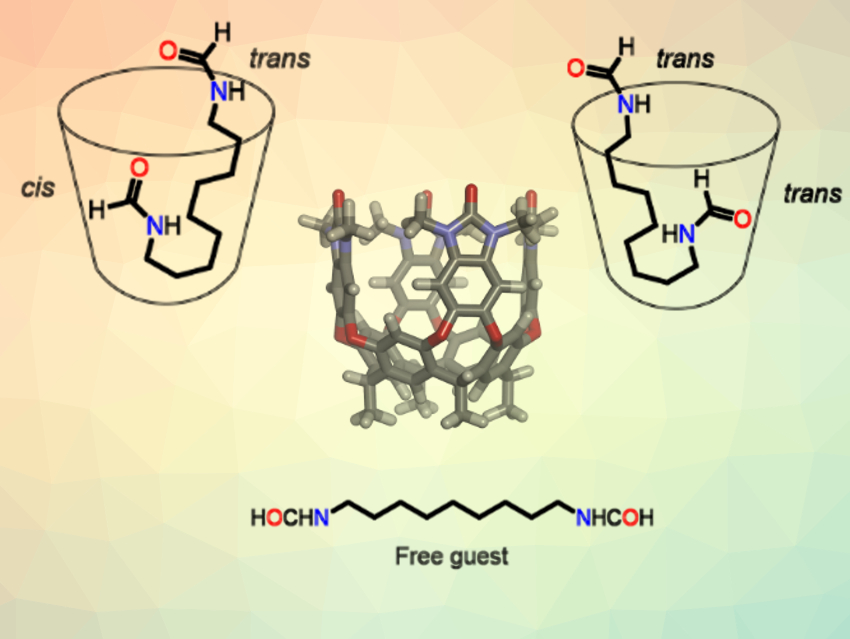
Yoram Cohen, Tel Aviv University, Israel, Yang Yu, Shanghai University, China, Julius Rebek Jr., Shanghai University and The Scripps Research Institute, La Jolla, CA, USA, Pablo Ballester, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain, and colleagues have used DOSY to study the kinetic stabilities and exchange dynamics of trans,trans- and trans,cis-bisformamide inclusion complexes (schematically pictured above).
These complexes, or “caviplexes”, consist of a long‐chain bis‐formamide as the guest (example pictured) and a water‐soluble, “vase-shaped” deep cavitand as the host (general 3D structure pictured above, chemical structure pictured below). The formamides have trans- and cis-isomeric forms, which can be interconverted by rotation around the amide’s C–N bond.
By performing DOSY experiments with different diffusion times, the team determined the kinetic stabilities of the isomeric trans,trans- and trans,cis-bisformamide complexes. The trans,cis-bisformamide isomers form kinetically and thermodynamically more stable complexes than their trans,trans-counterparts. Most likely, these differences arise from the different hydrophilicities of the cis- and trans-formamide ends of the guests.
- Kinetic Stabilities and Exchange Dynamics of Water‐Soluble Bis‐Formamide Caviplexes Studied Using Diffusion‐Ordered NMR Spectroscopy (DOSY),
Luis Escobar, Yong‐Sheng Li, Yoram Cohen, Yang Yu, Julius Rebek, Pablo Ballester,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202000781