Methods to chemically modify biomolecules are useful to study biological processes and, for example, develop new drugs. However, biomolecule functionalization can be challenging due to the need to function under physiological conditions and with low concentrations, as well as the required selective modification of the target without affecting its structure or function.
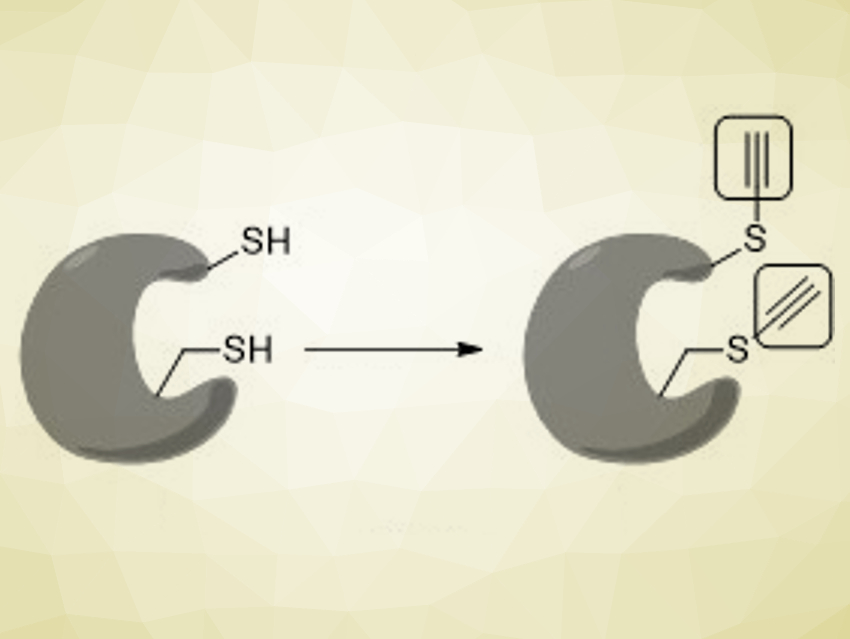
Guilhem Chaubet, University of Strasbourg, France, Alexander Adibekian, The Scripps Research Institute, Jupiter, FL, USA, Jerome Waser, Swiss Federal Institute of Technology (EPFL) Lausanne, Switzerland, and colleagues have developed a method for the ethynylation of thiol groups in the cysteine residues in peptides and proteins. An alkyne moiety is introduced selectively on cysteine using iodine-based ethynylbenziodoxolone (EBX) reagents (pictured below).
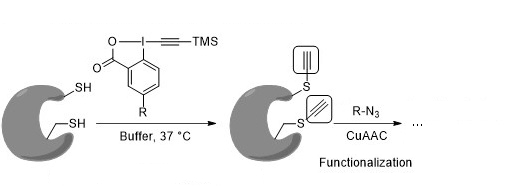
These reagents allowed the team to perform the desired ethynylations within minutes under physiological conditions. The installed alkynes are versatile biorthogonal groups that allow further functionalizations without interference with the biomolecules. Their small size ensures minimal structural disturbance.
The method was used for the modification of the antibody trastuzumab, an important anticancer drug. The approach was also used in living cells for modifying and identifying proteins that contain reactive cysteines. The reactivity and physical properties of the EBX reagents can be optimized by changing the substituent R.
- Ethynylation of Cysteines from Peptides to Proteins in Living Cells,
Romain Tessier, Raj Kumar Nandi, Brendan G. Dwyer, Daniel Abegg, Charlotte Sornay, Javier Ceballos, Stephane Erb, Sarah Cianferani, Alain Wagner, Guilhem Chaubet, Alexander Adibekian, Jerome Waser,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202002626