In the supercritical state, the properties of water are reversed: oils are dissolved, and salts precipitate. Cyril Aymonier, Institute of Condensed Matter Chemistry of Bordeaux (ICMCB), French National Center for Scientific Research (CNRS), Pessac, France, and colleagues have found a way to dissolve those precipitates: using a cosolvent made of molten salt that flows within the supercritical water fluid. This “hydrothermal molten salt” diphasic state could be useful in waste treatment or other applications where precipitate clogging causes problems.
Supercriticality: A Flowing, Dynamic State of Matter
Supercritical fluids represent a state of matter where liquid and gas phases coexist. Heating a substance above its critical temperature and compressing it above its critical pressure fluidizes it; its viscosity becomes very low, and its density highly variable. Although supercriticality might at first glance seem some exotic property of matter, supercritical fluids are used in a wide range of technologies across industries as varied as food processing, cleaning, coatings, and waste treatment. Water reaches its fluid state above 221 bar and 374 °C, at which point its polarity, surface tension, and viscosity drop and it becomes an aggressive oxidizing agent.
Its low polarity makes supercritical water a good solvent for organic compounds, and supercritical water oxidation is a common process in waste treatment and biomass reforming. However, what works with organic compounds is reversed with inorganic salts. Everything that is ionized and soluble in liquid water may clog tubes and valves in technological processes using supercritical water. Furthermore, it has been shown that corrosion rates are significantly higher beneath deposited solids.
However, not all salts are prone to precipitate out as solids. The team argued that electrolytes with a melting point below the critical temperature of water would precipitate when reaching their precipitation temperature, but at the melting point, the grains would fuse and melt to form a second, liquid phase within the supercritical water phase. This liquid phase could be useful as a cosolvent.
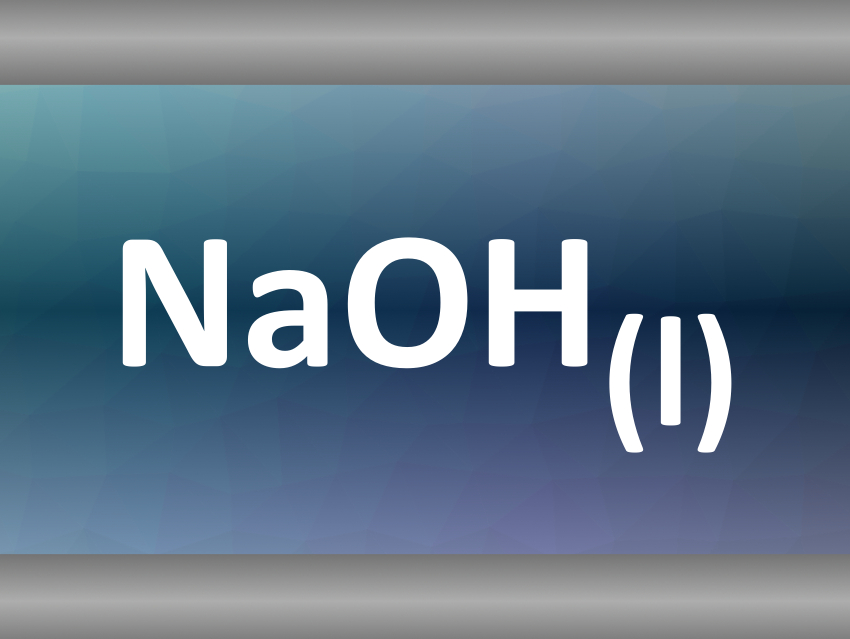
Sodium hydroxide, for example, melts at 318 °C. The scientists heated an aqueous sodium hydroxide solution in a sapphire capillary. When the system reached the precipitation temperature of NaOH, 380 °C, they observed the formation of liquid NaOH droplets. Because the precipitation temperature is higher than the melting temperature, the precipitation is directly followed by the fusion of the salt to give the molten droplets.
Molten Salt as a Cosolvent
Molten salts have been long used in other technologies, for example, as eutectic mixtures to dissolve inorganic materials, to melt and treat metals, as electrolytes, in fuel cells, and for oxidation and reduction processes. However, they are rarely used in continuous flow systems due to their viscosity and the technical hurdles of high-temperature injection, mixing, and handling the flows.
This could all change with a molten salt that forms a cosolvent in a supercritical fluid. The team proposes that such a diphasic system, which they called hydrothermal molten salt technology or HyMoS, may offer new ways of dissolving and processing inorganic materials, even in continuous systems. As a proof-of-principle, they injected an aqueous solution of sodium sulfate into a stream under pressure and heated it. As expected, the sodium sulfate precipitated from the mixture. In contrast, when sodium hydroxide was added, the grains disappeared and were found dissolved in NaOH droplets. After cooling down, the diphasic system resolved to give an alkaline aqueous solution of sodium sulfate.
The researchers conclude that HyMoS forms a suitable two-solvent system for supercritical water oxidation processes. It could represent a new and simple way to address the issue of clogging, which is at present usually addressed by installing elaborate reactor designs. Besides NaOH, other low-melting salts could also be investigated, though the team points out that sodium hydroxide offers advantages as a cheap, abundant, and thermally stable compound.
The researchers also propose that the two-phase supercritical fluid offers new opportunities for purely molten-salt technologies. They imagine designing hydrothermal water–salt emulsions with temperature- and pressure-tunable droplet structure for continuous applications in material synthesis, recycling, or catalysis.
- A new solvent system: Hydrothermal molten salt,
T. Voisin, A. Erriguible, C. Aymonier,
Sci. Adv. 2020, 6, eaaz7770.
https://doi.org/10.1126/sciadv.aaz7770