Petrochemical conversion processes that convert alkanes to value-added products often require harsh reaction conditions and expensive metal catalysts, while sometimes providing only poor product selectivities. In contrast to these traditional industrial processes, enzymes can catalyze chemical reactions under mild conditions with unparalleled selectivity. Using redox enzymes in an enzymatic fuel cell can combine the catalysis of industrially useful reactions, such as the oxyfunctionalization of alkanes, with the simultaneous production of electricity.
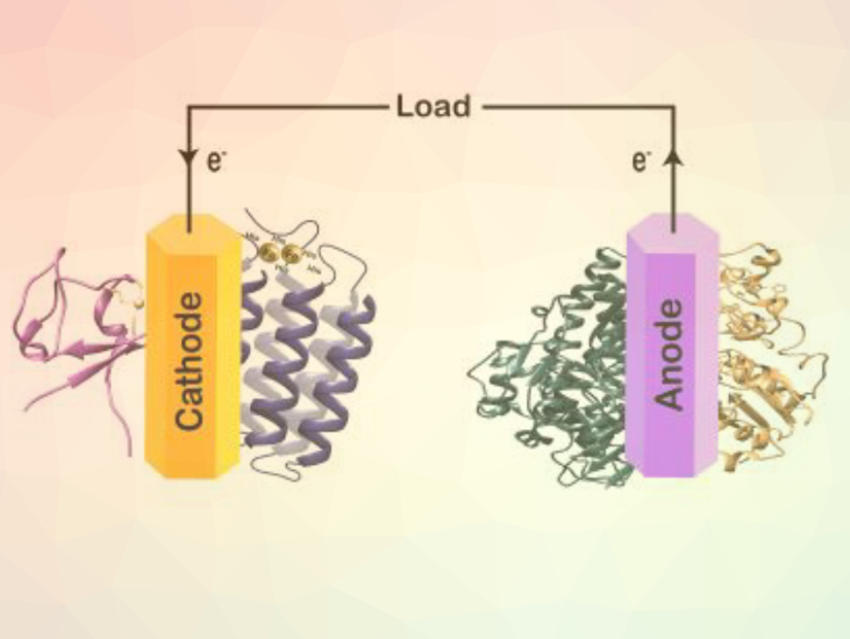
Shelley D. Minteer, University of Utah, Salt Lake City, USA, and colleagues have designed an electrode for application in enzymatic fuel cells. The diiron enzyme alkane monooxygenase Pseudomonas Putida (alkB) selectively oxidizes the terminal carbon atoms of alkanes. AlkB was combined with the electron-transfer protein rubredoxin to form a biocathode. Toluidine blue O (TBO) was used as an electrochemical mediator to continuously supply electrons to the biocathode enzymes.
The biocathode can be used in different oxyfunctionalization reactions to produce alcohols, epoxides, or sulfoxides. 1-Octanol, for example, was produced from octane with a Faradaic efficiency of 25 %. The cathode was combined with a hydrogenase bioanode in an enzymatic fuel cell. Oxidizing H2 as a clean fuel, this fuel cell can then generate power while producing alcohols, epoxides, or sulfoxides. According to the researchers, this bioelectrochemical system represents an alternative, environmentally conscientious approach to petrochemical conversions with high added value.
- Selective Electroenzymatic Oxyfunctionalization by Alkane Monooxygenase in a Biofuel Cell,
Mengwei Yuan, Sofiene Abdellaoui, Hui Chen, Matthew J. Kummer, Christian A. Malapit, Chun You, Shelley D. Minteer,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202003032