The optimization of drug candidates in medicinal chemistry often involves the exploration of many possible substitution patterns. Diazo compounds can be particularly useful for this. Expanding the available types of diazo reagents, thus, could allow easy access to a wider range of the chemical space—potentially including promising drug candidates.
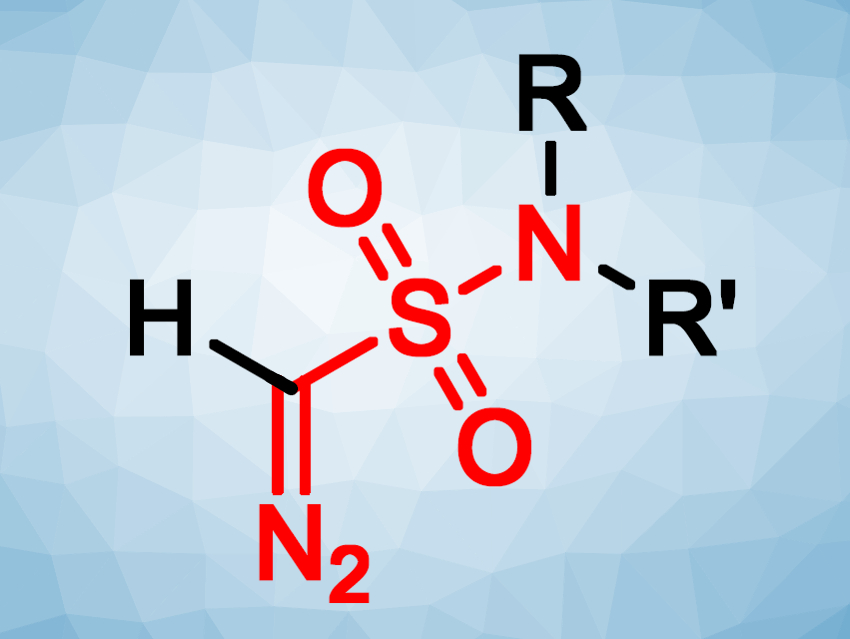
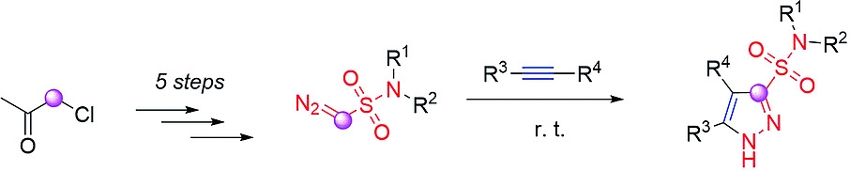
Mikhail Krasavin and colleagues, Saint Petersburg State University, Russia, have developed a new type of diazo reagents, CH–diazomethane sulfonamides (pictured above). The compounds were prepared via a five-step synthesis starting from chloroacetone. Chloroacetone was reacted with sodium sulfite in water to give a sodium sulfonate. This intermediate was converted to the respective sulfonyl chloride using (COCl)2 in acetonitrile at room temperature. Reactions with different secondary amines then yielded sulfonamide derivatives. The so-called “sulfonyl-azide-free” (or “SAFE”) protocol, with a reactant mixture consisting of 3-(chlorosulfonyl)benzoic acid, NaN3, and K2CO3, was then used to introduce the diazo group. Finally, the acetyl group was removed using excess Al2O3 in dichloromethane.
The resulting reagents carry a sulfonamide group, which can be useful in biologically active compounds, and a diazomethane moiety, which can enter various cycloaddition reactions. As an example, the developed diazo reagents can react with alkynes and their synthetic equivalents via a [3+2] addition to give pyrazole sulfonamides (pictured below).
- Synthetic studies towards CH-diazomethane sulfonamides: a novel type of diazo reagents,
Andrey Bubyrev, Dmitry Dar’in, Grigory Kantin, Mikhail Krasavin,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000446