Calcium-metal batteries (CMBs) have the potential to provide high-capacity, cost-effective energy storage. They could be an alternative to state-of-the-art lithium-ion batteries. However, currently available CMBs suffer from short lifespans due to the poor reversibility of the calcium-metal anode. There is a lack of suitable electrolytes for reversible Ca plating/stripping at room temperature.
Ruiguo Cao, Shuhong Jiao, University of Science and Technology of China, Hefei, Guohua Tao, Peking University Shenzhen Graduate School, Shenzhen, China, and colleagues have developed an electrolyte that improves the reversibility of Ca‐metal anodes at room temperature. The team used a nonaqueous dual electrolyte composed of Ca(BH4)2 and LiBH4 in tetrahydrofuran (THF). Using this approach, they achieved high Coulombic efficiencies of up to 99.1 % for the Ca plating/stripping processes and a stable long-term cycling performance for over 200 cycles.
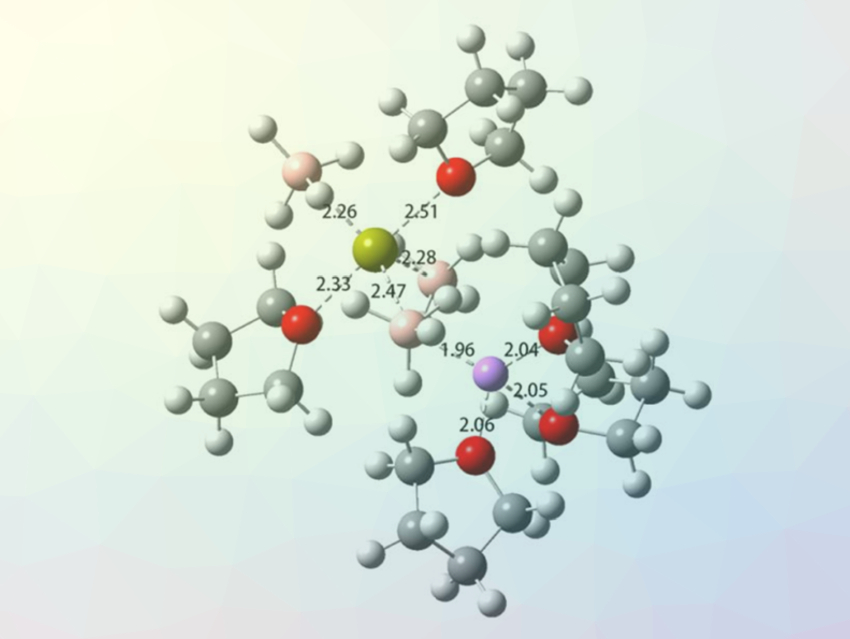
According to the researchers, the improved battery properties are caused by a change in the electrolyte solvation structure. NMR spectra and molecular dynamics (MD) simulations showed that the presence of Li+ ions decreases the oxygen coordination number in the first solvation shell of the Ca2+ ions—i.e., the number of solvent molecules. This makes the desolvation of Ca2+ at the electrode–electrolyte interface faster. Since the desolvation process is considered the rate‐determining step for electroplating, this improves the Ca plating/stripping process.
- Electrolyte Solvation Manipulation Enables Unprecedented Room-Temperature Calcium-Metal Batteries,
Yulin Jie, Yunshu Tan, Linmei Li, Yehu Han, Shutao Xu, Zhenchao Zhao, Ruiguo Cao, Xiaodi Ren, Fanyang Huang, Zhanwu Lei, Guohua Tao, Genqiang Zhang, Shuhong Jiao,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202002274