Hybrid halide materials consist of a cationic organic amine (A), a metal (M), and a halide (X). Some of these AMX3 perovskites are interesting magnetic materials. AMX3 perovskites and hybrid tin and lead halides have revolutionized solar cells by allowing their low-cost, simple production.
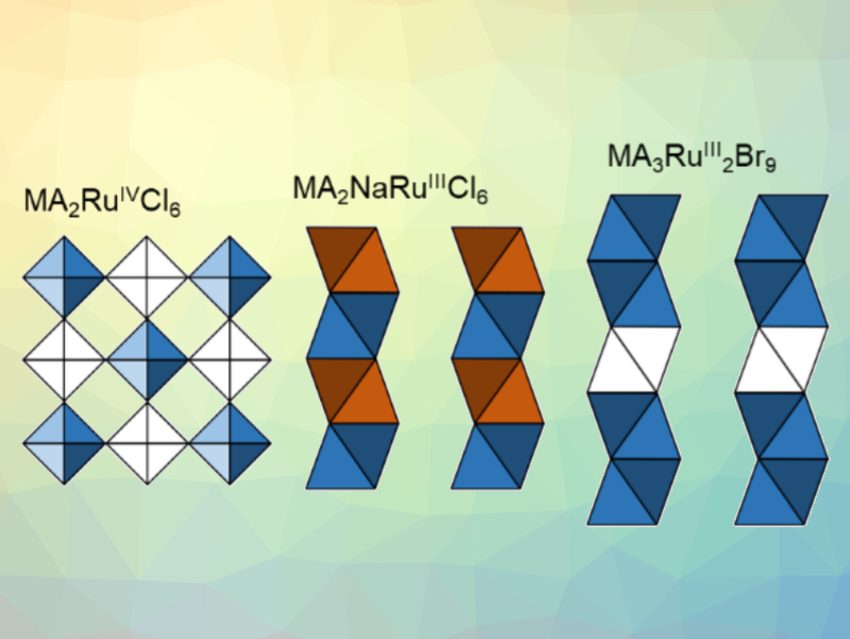
Ram Seshadri, Anthony Cheetham, and colleagues, University of California, Santa Barbara, USA, have synthesized a range of new methylammonium (MA) ruthenium halides. The compounds were prepared solvothermally starting from RuCl3. Hypophosphorous acid was used to control the oxidation state of ruthenium. The three classes of compounds prepared, MA2RuIVX6, MA2MRuIIIX6, and MA3RuIII2Br9 (M = Na, K, or Ag; X = Cl, Br; examples pictured), crystallize in different structures, as shown by single-crystal X-ray diffraction.
The compounds of the type MA2RuX6 (pictured left) crystallize in the trigonal space group R3m. The compounds of the type MA2MRuX6 (pictured center) crystallize in a structure related to BaNiO3, with alternating MX6 and RuX6 octahedra forming chains in the trigonal P3m space group. The compound MA3Ru2Br9 (pictured right) crystallizes in the orthorhombic Cmcm space group and contains pairs of face‐sharing octahedra. The magnetic behavior of all three classes of compounds is influenced by spin-orbit coupling, and the effective magnetic moments of the compounds decrease in the order MA2RuX6 > MA2MRuX6 > MA3Ru2Br9.
- Structural Diversity and Magnetic Properties of Hybrid Ruthenium Halide Perovskites and Related Compounds,
Pratap Vishnoi, Julia L. Zuo, T. Amanda Strom, Guang Wu, Stephen D. Wilson, Ram Seshadri, Anthony K. Cheetham,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202003095