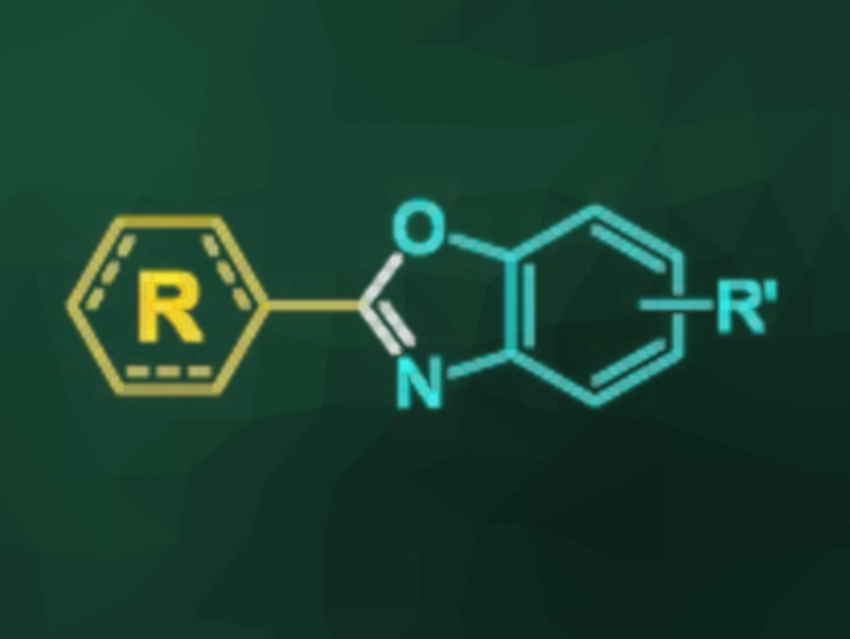
Benzoxazoles are useful molecules in organic synthesis and medicinal chemistry due to their reactivities and bioactivities. They can be synthesized from 2-aminophenols by condensation with carboxylic acid derivatives or by oxidative coupling. The latter strategy requires using an oxidant in the presence of a catalyst. Dioxygen can be used as a green oxidant. However, it has drawbacks such as the requirement for a transition-metal catalyst, possible overoxidation, a high reaction volume and/or pressure, as well as handling difficulties due to its gaseous state. As a heavier oxygen congener, sulfur could be a cheap and versatile alternative oxidant for this transformation.
Thanh Binh Nguyen, Institut de Chimie des Substances Naturelles, Gif-sur-Yvette, France, and Vietnam Academy of Science and Technology, Hanoi, Quoc Anh Ngo, Vietnam Academy of Science and Technology, and colleagues have developed a simple synthesis of benzoxazoles. The desired heterocycles were prepared by heating equimolar amounts of aldehydes with 2-aminophenols and sulfur in the presence of Na2S·5H2O as a catalyst in the presence of dimethylsulfoxide (DMSO) at 70 °C (reaction pictured below).
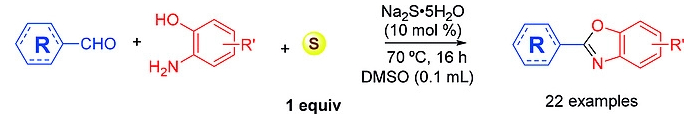
The protocol has a good functional group tolerance and can be used with both aromatic and aliphatic aldehydes. The desired products were obtained in moderate to good yields. The reaction avoids the downsides of similar protocols that use oxygen as the oxidant.
- Sulfur-promoted Synthesis of Benzoxazoles from 2-Aminophenols and Aldehydes,
Le Anh Nguyen, Thai Duy Dang, Quoc Anh Ngo, Thanh Binh Nguyen,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000523