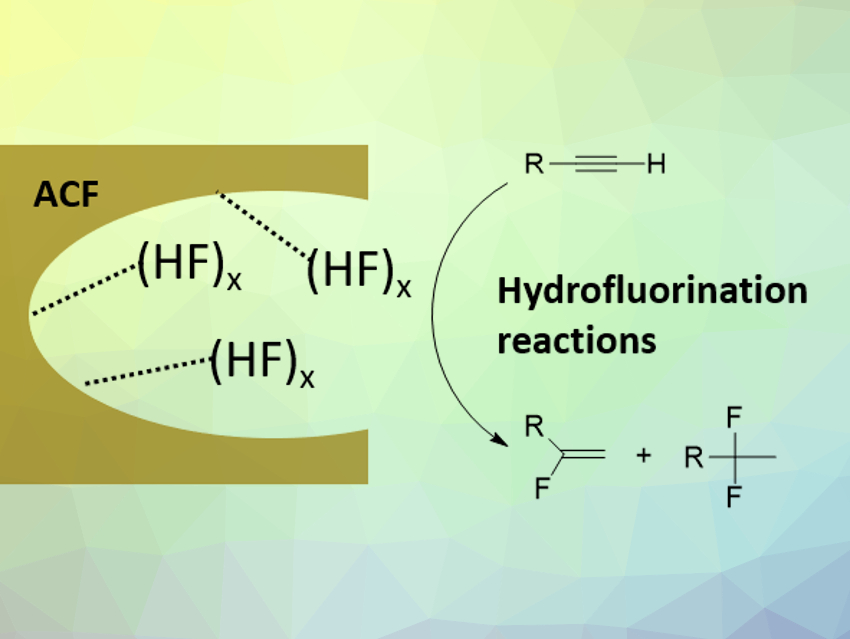
Highly Lewis-acidic, nanoscopic, amorphous aluminum chlorofluoride (ACF) is a useful catalyst for a variety of chemical conversions. It can, for example, catalyze C-F bond activation reactions, such as hydrodefluorination or dehydrofluorination processes. Hydrofluorination reactions represent are routes to fluorinated building blocks, which have applications, e.g., in pharmaceutical or materials science.
Thomas Braun, Humboldt University Berlin, Germany, and colleagues have developed a procedure to immobilize anhydrous HF onto the surface of ACF and investigated the activity of the resulting HF‐loaded ACF for the hydrofluorination of alkynes. The team first condensed freshly dried HF onto ACF. Then they applied a vacuum for 15 min to remove any excess HF that did not interact with the surface.
The HF-loaded ACF was characterized using magic-angle spinning (MAS) NMR spectroscopy, Fourier-transform infrared (FTIR) spectroscopy, X-ray diffraction, and inelastic neutron scattering (INS), among other methods. The team found that HF was successfully immobilized on the surface of the ACF, forming polyfluoride structures. The Lewis and Brønsted acidity of the material were strongly reduced, presumably due to interactions between HF and the surface. Thus, loading HF onto the surface of ACF does not result in the generation of a superacid similar to, e.g., SbF5/HF. However, the loaded HF can be transferred to alkynes in hydrofluorination reactions under mild conditions to give mono- or difluorinated alkenes (pictured above on the right).
- A HF Loaded Lewis‐Acidic Aluminium Chlorofluoride for Hydrofluorination Reactions,
Maëva‐Charlotte Kervarec, Erhard Kemnitz, Gudrun Scholz, Svemir Rudić, Thomas Braun, Christian Jäger, Adam A. L. Michalchuk, Franziska Emmerling,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202001627