Interactions between two or more proteins—protein-protein interactions (PPIs)—regulate virtually all cellular processes. PPIs are important in disease development and progression, and thus, can be promising drug targets. So-called peptide “stapling” can be used as a method for targeting α-helix mediated PPIs, i.e., those in which binding is based on the docking of an α-helix from one protein into a cleft on another. In such a “stapling” approach, a peptide is constrained in an α-helical (or similar) conformation to enhance its target-binding affinity.
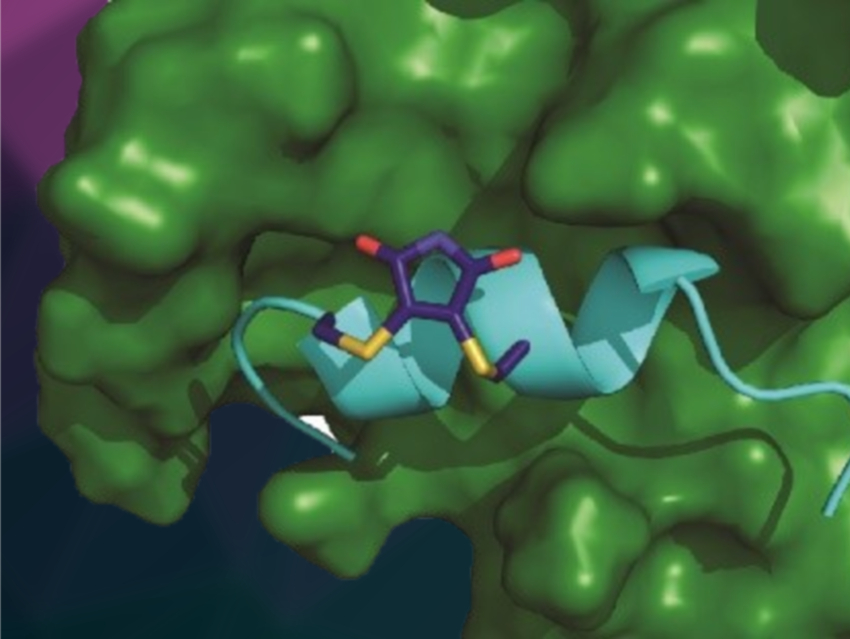
Andrew J. Wilson, University of Leeds, UK, and colleagues have “stapled” peptides derived from hypoxia‐inducible factor 1 (HIF‐1α) into a constrained configuration. Their aim was to target the interaction between HIF-1α and the protein p300, which involves an α-helix. The HIF-1α/p300 interaction plays a key role in tumor metabolism and is a promising anticancer target. The team used a reversible dibromomaleimide stapling to connect cysteine residues on HIF-1α peptides to pre-organize them and support the formation of an α-helix.
This “stapling” led to increased binding affinity of the peptide to p300 (simulated complex pictured) compared with the unconstrained version. This observed increase is not directly caused by an increased population of an α‐helical conformation in the unbound state. Rather, it is due to an improved ability of the peptide to adopt a bioactive α‐helical conformation in the bound state, which is supported by the constrained configuration. According to the researchers, these results could help to improve the design of peptides that target therapeutically important proteins.
- Stapled Peptides as HIF‐1α/p300 Inhibitors: Helicity Enhancement in the Bound State Increases Inhibitory Potency,
Kristina Hetherington, Zsofia Hegedus, Thomas A. Edwards, Richard B. Sessions, Adam Nelson, Andrew J. Wilson,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202000417