Our immune system ought to be able to recognize and kill tumor cells. However, many tumors deceive the immune system. For example, they induce the so-called immune checkpoints of T-cells to shut down immune responses. Lei Liu, Tsinghua University, Beijing, China, Yanfeng Gao, Zhengzhou University and Sun Yat-sen University, Shenzhen, both China, and colleagues have introduced a new approach for immunological tumor treatment. Their method is based on the specific blockade of an immune checkpoint by a stable “mirror-image” peptide.
Cancer Immunotherapy
T lymphocytes have a variety of immune checkpoints on their surface, some that crank up the immune system and others that suppress immune reactions when they discover suitable ligands on the surfaces of “checked” cells. One such immune checkpoint is the programmed cell death protein 1 (PD-1). If the PD-L1 ligand is bound to PD-1, the immune response is inhibited to prevent attacks on healthy cells produced by the body. Unfortunately, many tumors “camouflage” themselves with a particularly large number of PD-L1, which protects them. Blocking the interaction between PD-1 and PD-L1 can normalize the cancer immunity in the microenvironment around tumors. However, previous therapeutic approaches had only limited success, and the tumors often developed resistance.
A newly discovered immune checkpoint known as TIGIT could provide an alternative point of attack. TIGIT reacts to a ligand named PVR with an immunosuppressive signal. The team used RNA expression data from the Cancer Genome Atlas and Gene Expression Omnibus dataset to discover that TIGIT is much more common than PD-1 in many tumors, including those resistant to anti-PD-1 therapy.
Checkpoint Blockade by a Peptide
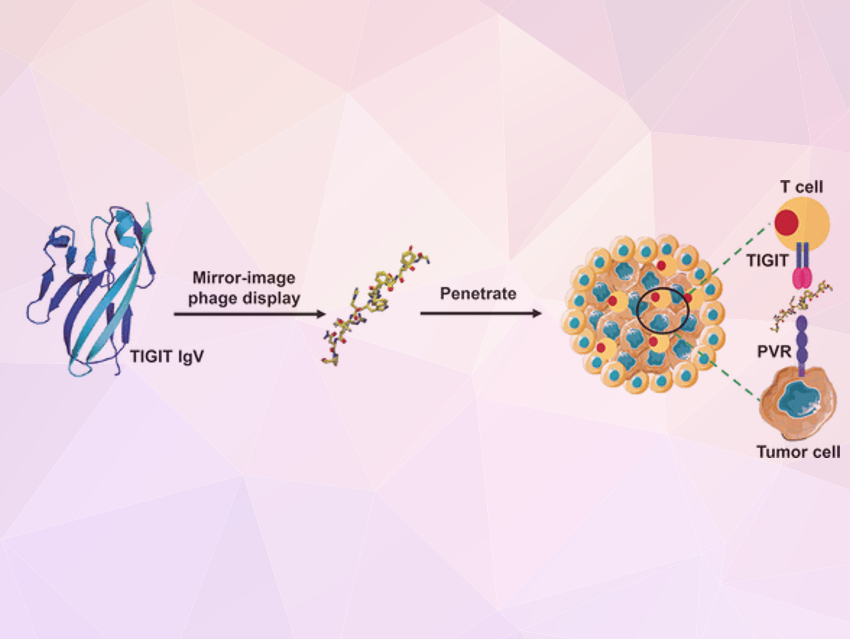
The researchers wanted to use a peptide as their new drug because these molecules penetrate more deeply into tissue with affinities and specificities equal to those of antibodies. They cause significantly fewer undesired immunological side effects and are easier to produce. Their disadvantage is that they are rapidly broken down by proteases in the body. For this reason, the researchers decided to use “mirror-image” peptides, which are stable toward proteases. Amino acids can exist in the natural L-configuration, or its mirror image, the synthetic D-configuration. D-peptides made from D-amino acids are significantly more long-lived than L-peptides.
To find a suitable peptide, the researchers used the mirror-image phage display technique. In this method, very large numbers of different biotechnologically produced peptides are presented on the surfaces of phages (viruses that attack bacteria). Those that bind to the desired target molecule are then selected and multiplied in bacteria. They then go through further selection cycles until only very strongly binding peptides remain. Initially, L-peptides are presented in mirror-image phage display. However, those selected bind to the mirror image of the target molecule. For this, the researchers synthesized a portion of TIGIT in the D-configuration. As the last step, they produced the mirror-image D-version of the strongest binding L-peptide, which fitted the key binding interface of the TIGIT/PVR protein perfectly.
The D-peptide known as DTBP-3 developed by this technique effectively blocks the interaction of TIGIT with PVR, is stable toward proteases, and inhibits the growth and metastasis of anti-PD-1 resistant tumor models.
- A Novel D‐Peptide Identified by Mirror‐Image Phage Display Blocks TIGIT/PVR for Cancer Immunotherapy,
Xiuman Zhou, Chao Zuo, Wanqiong Li, Weiwei Shi, Xiaowen Zhou, Hongfei Wang, Shaomeng Chen, Jiangfeng Du, Guanyu Chen, Wenjie Zhai, Wenshan Zhao, Yahong Wu, Yuanming Qi, Lei Liu, Yanfeng Gao,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202002783