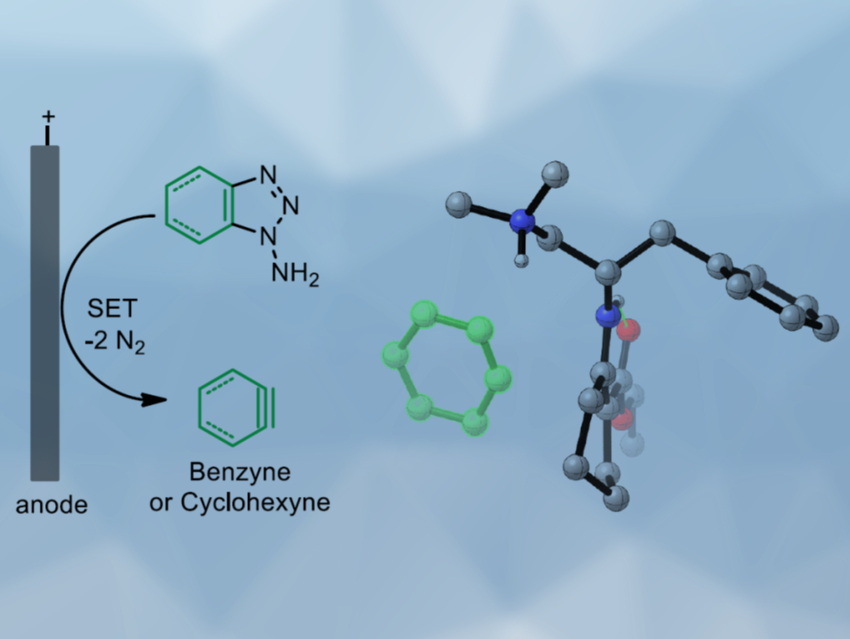
Benzyne is a reactive intermediate whose structure can be derived from benzene by removing two hydrogen atoms. Generally, benzyne is generated in situ and reacts spontaneously with nucleophilic compounds. Catalytic reactions with benzyne are challenging due to its high reactivity and low polarity. Specifically, asymmetric catalysis involving a benzyne intermediate had not been achieved so far.
Sanzhong Luo, Tsinghua University, Beijing, China, and colleagues have developed an electrochemical approach for the generation of benzyne intermediates (pictured above). The team used 1-aminobenzotriazole as a benzyne precursor. The electrolysis was performed at room temperature in acetonitrile using platinum electrodes in an undivided cell. The in-situ generated benzyne was trapped in a chiral enamine intermediate to allow the enantioselective arylation of β-ketocarbonyls (reaction pictured below, trapping process pictured in the animated graphic). The team used a chiral primary aminocatalyst derived from L-phenylalanine (pictured in blue). Cobalt acetate was used to stabilize the arynes and facilitate coupling with the enamine.
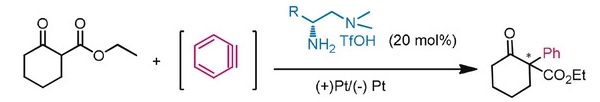
The reaction gives α-aryl cyclic β-ketocarbonyls with all-carbon-substituted stereogenic centers in good enantioselectivities. The developed catalytic protocol could also be used for couplings with cyclohexyne, another strained cyclic alkyne species.
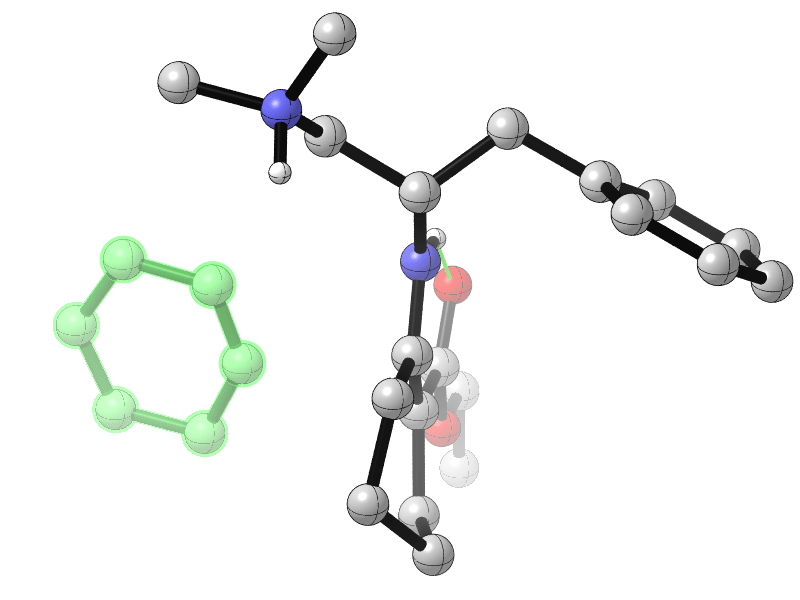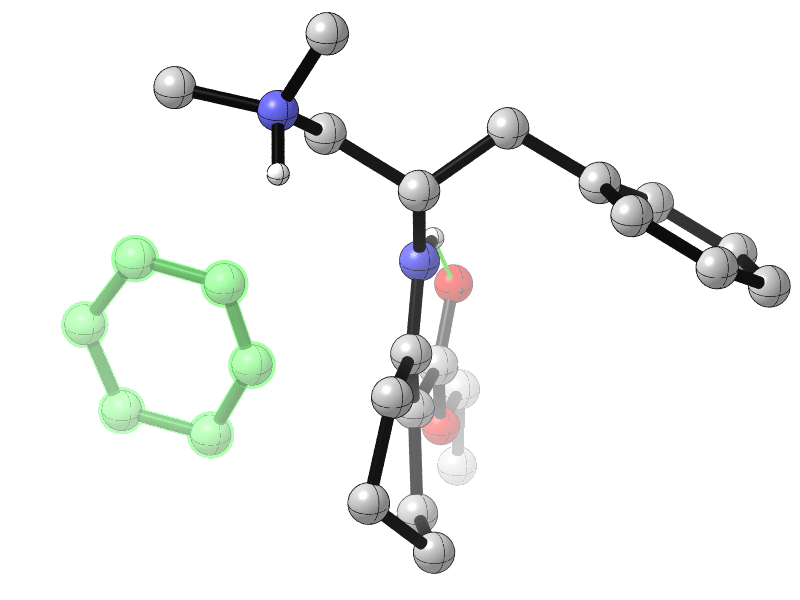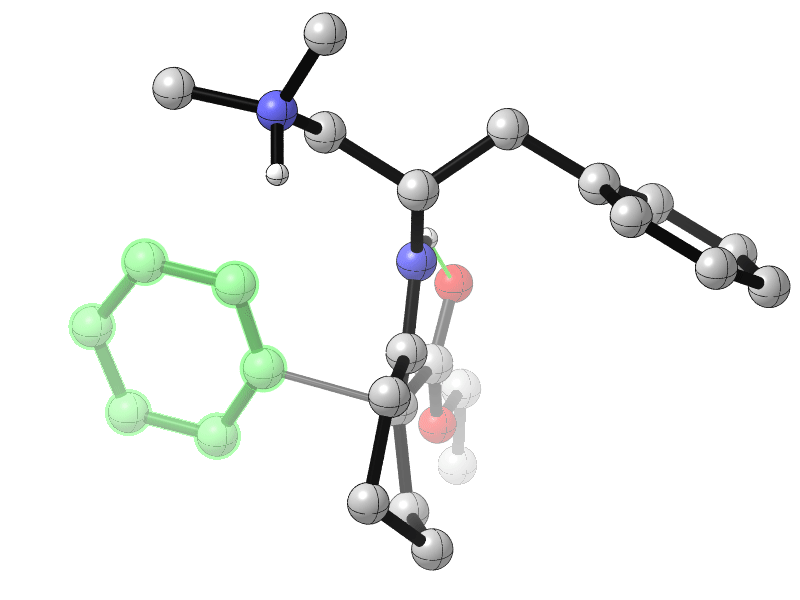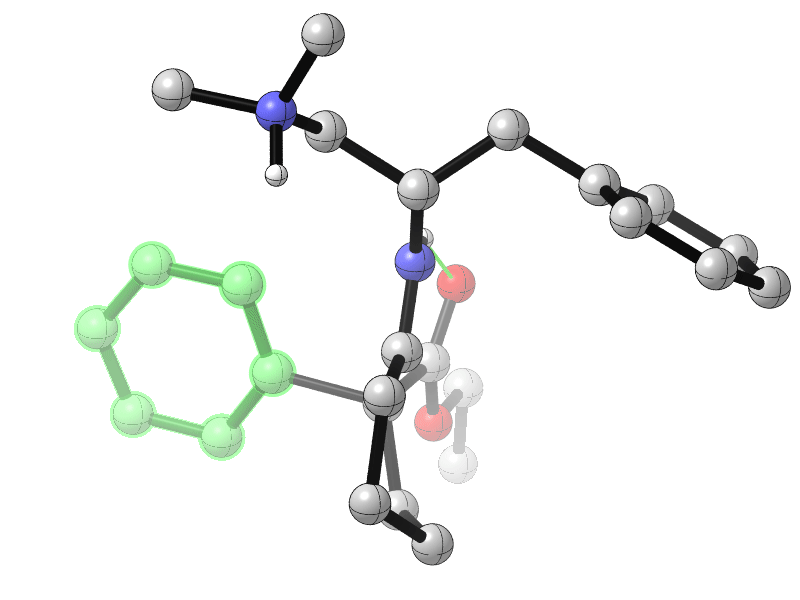
- Catalytic Asymmetric Electrochemical α-Arylation of Cyclic β-Ketocarbonyls with Anodic Benzyne Intermediates,
Longji Li, Yao Li, N Fu, L Zhang, Sanzhong Luo,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202006016