Controlling the dissociation kinetics of host–guest complexes is useful for their application, e.g., in biomedicine, drug delivery, catalysis, or sensing. Cucurbit[7]uril (CB7) has particularly high potential in these fields. For cucurbituril-based host–guest complexes, it is known that the apparent binding constant of guest encapsulation can be reduced in the presence of inorganic salts. However, the details of the salt-promoted guest release from the cavity of cucurbituril are not well understood.
László Biczók, Research Centre for Natural Sciences, Budapest, Hungary, and colleagues have found two contrasting mechanisms for the cation-induced guest release from CB7. The team monitored the guest release after the dilution of solutions of two different host–complexes by measuring changes in fluorescence intensity. One complex contained the symmetric dicationic 2,7-dimethyldiazapyrenium (MDAP2+) as a guest, the other the monocationic, non-symmetric berberine (B+, pictured below in red), a natural alkaloid. The measurements were performed at different concentrations of cations such as K+, Ca2+, or Ba2+.
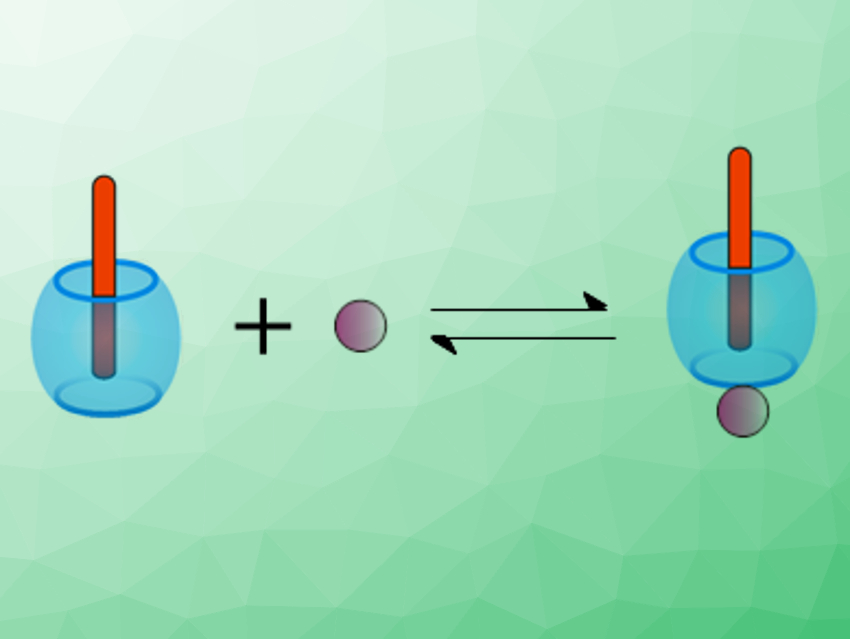
A salt-independent rate was found for the exit of MDAP2+ from the complex. In contrast, berberine was expelled in an unprecedented multistep process via ternary complex formation with a cation (pictured below), which the researchers could deduce from the observed kinetics. The results could help with the development of new stimuli-responsive assemblies and motivate systematic kinetic investigations of other host–guest systems.
.jpg)
- Kinetics and Mechanism of Cation‐Induced Guest Release from Cucurbit[7]uril,
Zsombor Miskolczy, Mónika Megyesi, László Biczók, Amrutha Prabodh, Frank Biedermann,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201905633



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)