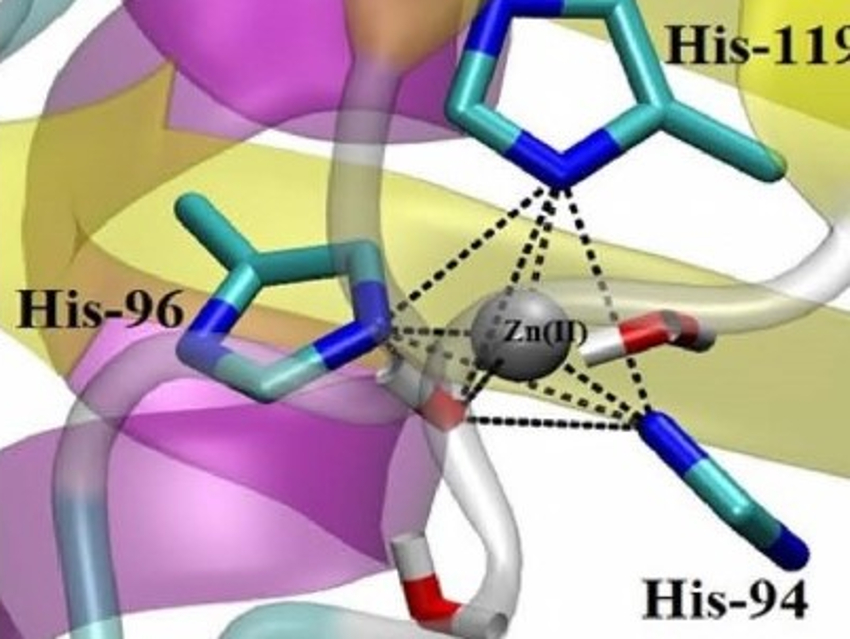
Human carbonic anhydrase (HCA) II is a very efficient enzyme. It has been studied extensively as a prototype in biophysics, biochemistry, inhibitor design, and medicinal chemistry. It has a high thermal stability and helps to maintain the pH balance in our body. HCA II catalyzes the reversible hydration of carbon dioxide and contains a stable, tetrahedrally coordinated catalytic zinc site (pictured).
Tanmoy K. Paul and Srabani Taraphder, Indian Institute of Technology, Kharagpur, have studied how changes in the coordination number of the zinc ion affect the rate-determining step in HCA II. This reaction step involves a proton transfer between a zinc-bound water and a histidine (His) residue in the enzyme. The team used molecular dynamics (MD) and transition path sampling simulations to study the effects of the zinc ion’s coordination environment, starting from a high-resolution crystal structure.
The researchers found that in addition to the stable tetrahedral state, there is a less stable, pentacoordinated, trigonal bipyramidal state, in which an extra water molecule transiently binds to the tetracoordinated zinc ion at the active site of HCA II. The deprotonation of a zinc-bound water molecule preferentially takes place from this transient penta‐coordinated state of zinc. It then sets in motion an intramolecular proton transfer via other water molecules to the His‐64 sidechain, which points inward to the active site (pictured). The rare constant estimated on this basis matches the experimental value, which suggests that dynamic Zn coordination triggers the rate-determining proton-transfer step in HCA II.
- Coordination Dynamics of Zinc Triggers the Rate Determining Proton Transfer in Human Carbonic Anhydrase II,
Tanmoy Kumar Paul, Srabani Taraphder,
ChemPhysChem 2020.
https://doi.org/10.1002/cphc.202000177