Benzene is the prototype for aromatic molecules. The concept of planar, benzene-like aromaticity can be extended to three-dimensional systems that feature so-called spherical aromaticity. One example of such a three-dimensional aromatic compound is the icosahedral closo-[B12H12]2−.
Planar and spherical aromatic compounds follow different electron rules. However, a common property is their ability to sustain a long-range shielding cone under an external magnetic field. This cone accounts for the nuclear shielding of neighboring atoms or molecules and is relevant for studying processes such as intermolecular aggregation by nuclear magnetic resonance.
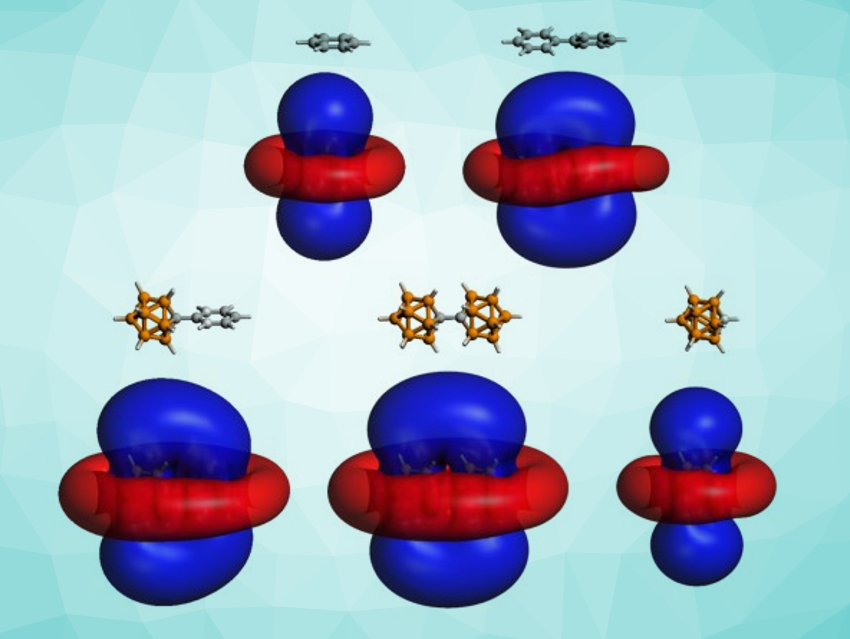
Alvaro Muñoz-Castro, Universidad Autonoma de Chile, Santiago, has studied bridged planar–planar, planar–spherical, and spherical–spherical aromatic species to understand the interplay between planar and spherical aromaticity. The team performed density functional theory (DFT) calculations to study the magnetic shielding of biphenyl (planar–planar system), [Ph–CB11H11]− (planar–spherical system), and [CB11H11]22− (spherical–spherical system).
The team found that these dual systems show two separate aromatic regimes with independent shielding cones. Isosurfaces for the induced magnetic fields in the three dual compounds, as well as free benzene and [CHB11H11]−, are shown above (external magnetic field perpendicular to the phenyl rings/the central bond). The cones of the two components share common shielding regions. The results can be extended to networks involving multiple independent aromatic states. They could provide new insights into the behaviors of different aromatic species when they are mixed, bridged, or interacting via non-covalent interactions.
- Interplay Between Planar and Spherical Aromaticity: Shielding Cone Behavior in Dual Planar‐Planar, Planar‐Spherical and Spherical‐Spherical Aromatics,
Alvaro Muñoz‐Castro,
ChemPhysChem 2020.
https://doi.org/10.1002/cphc.202000322