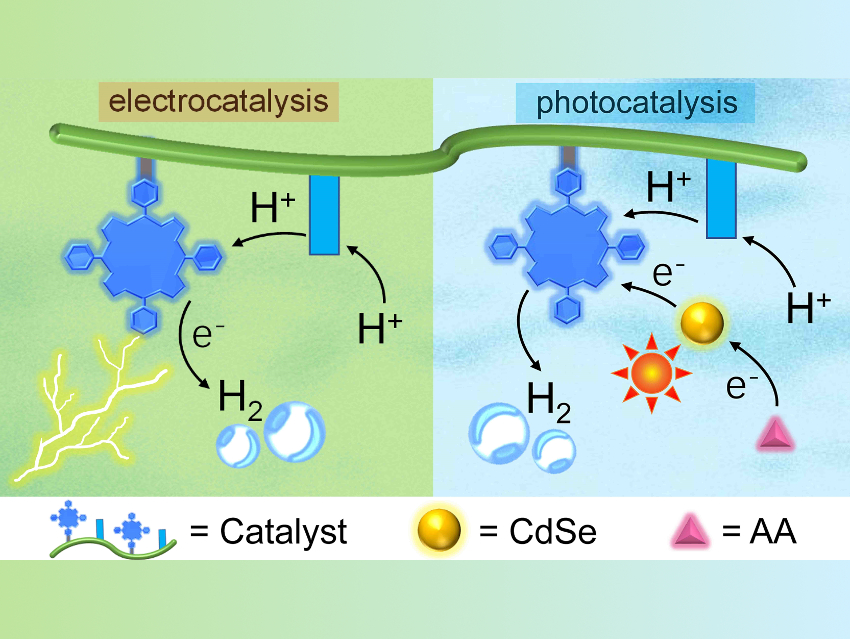
Water splitting can be used to create clean hydrogen fuel from water. This technology depends on efficient catalysts for the hydrogen evolution reaction (HER). Several metal complexes are active for the HER. However, most of these catalysts have limited solubility in water and require the use of organic solvents, as well as additional organic acids to provide protons.
Weian Zhang, East China University of Science and Technology, Shanghai, Rui Cao, Shaanxi Normal University, Xi’an, China, and colleagues have developed a strategy to solubilize HER catalysts in water, which then serves as both solvent and proton source. The team linked cobalt porphyrins, which are highly active HER catalysts, to water-soluble polymers with different side-chains (pictured below).
The team used a copolymerization of methacrylate-functionalized free-base tetraphenylporphyrin with methacrylic acid, followed by a reaction with cobalt nitrate to give Co-1 (pictured below). Replacing methacrylic acid with 2-(dimethylamino)ethyl methacrylate gave Co-2, and Co-3 was synthesized using oligo(ethylene glycol) methyl ether methacrylate.
.jpg)
The polymers display high efficiency and stability in neutral aqueous solutions for both electro- and photocatalytic HER (pictured at the top). For the photocatalytic HER, the team used CdSe as the photosensitizer and ascorbic acid (AA) as the sacrificial electron donor. The side-chains of the polymers can mimic activity-controlling residues in enzymes and tune the catalytic performance. The electrocatalytic HER activity trend is Co-1 > Co-2 > Co-3, while the photocatalytic HER activity trend is Co-2 > Co-1 > Co-3. According to the team, this bioinspired strategy to improve solubility and tune activity might also be useful for other molecular catalysts.
- Water-Soluble Polymers with Appending Porphyrins as Bioinspired Catalysts for the Hydrogen Evolution Reaction,
Lisi Xie, Jia Tian, Yingjie Ouyang, Xinai Guo, Weian Zhang, Ulf-Peter Apfel, Wei Zhang, Rui Cao,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202003836