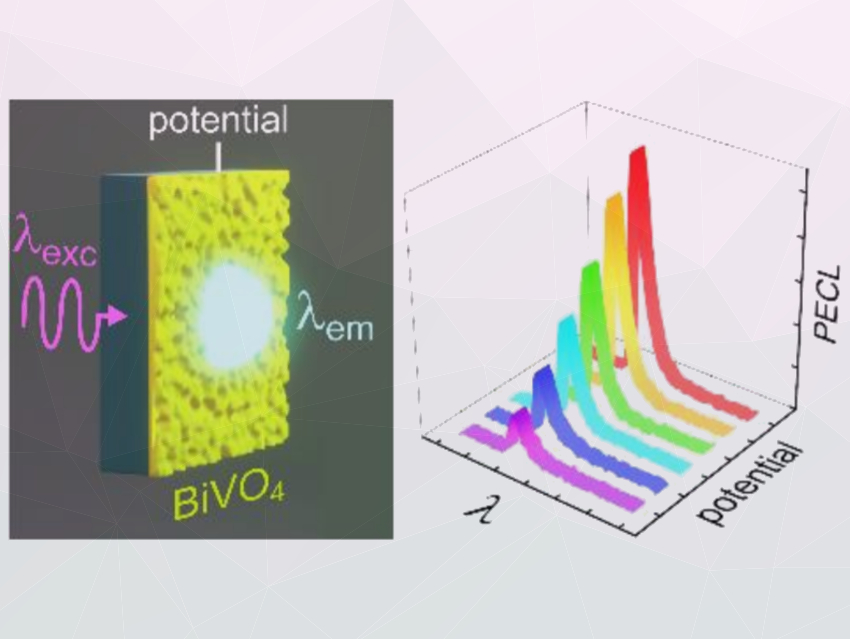
Photoelectrochemistry (PEC) is electrochemistry that occurs at the interface of an illuminated semiconductor and an electrolyte. Electrochemistry can also be used to generate light in a phenomenon known as electrochemiluminescence.
These two fields can be combined in photoinduced electrochemiluminescence (PECL), where light is converted into a different wavelength by an electrochemical reaction at a semiconductor photoelectrode and allows the triggering of electroluminescence at low potentials. However, PECL systems often have problems caused by photoelectrochemical instability of the semiconductor electrodes. BiVO4 is a promising material for photoanodes due to its electronic and optical properties, stability, and nontoxicity.
Neso Sojic, University of Bordeaux, France, Gabriel Loget, University of Rennes, France, and colleagues have designed a system that can amplify the light emission of a fluorescent solution based on PECL of a luminol derivative. Thin BiVO4 layers were deposited on a fluoride-doped SnO2 (FTO) substrate and then used as the photoanode in a PEC cell with an aqueous electrolyte containing the luminol derivative 8‐amino‐ 5‐chloro‐2,3‐dihydro‐7‐phenyl‐pyrido[3,4‐d]pyri‐dazine‐1,4‐dione.
The researchers illuminated the cell with a light-emitting diode (LED) located below the FTO, and they recorded emission spectra with an optical fiber placed above the electrolyte. The system was subjected to an electric field and light illumination simultaneously (pictured), thereby inducing luminescence in the fluorescent solution. Photoinduced electrochemiluminescence (PECL) occurred at potentials as low as –0.4 V and was stable for several hours. The PECL effect can be used to finely tune the luminescence of the luminol derivative. According to the researchers, this concept could have applications in biosensing and light‐addressable devices.
- Luminescence Amplification at BiVO4 Photoanodes by Photoinduced Electrochemiluminescence,
Jing Yu, Hiba Saada, Rawa Abdallah, Gabriel Loget, Neso Sojic,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202004634