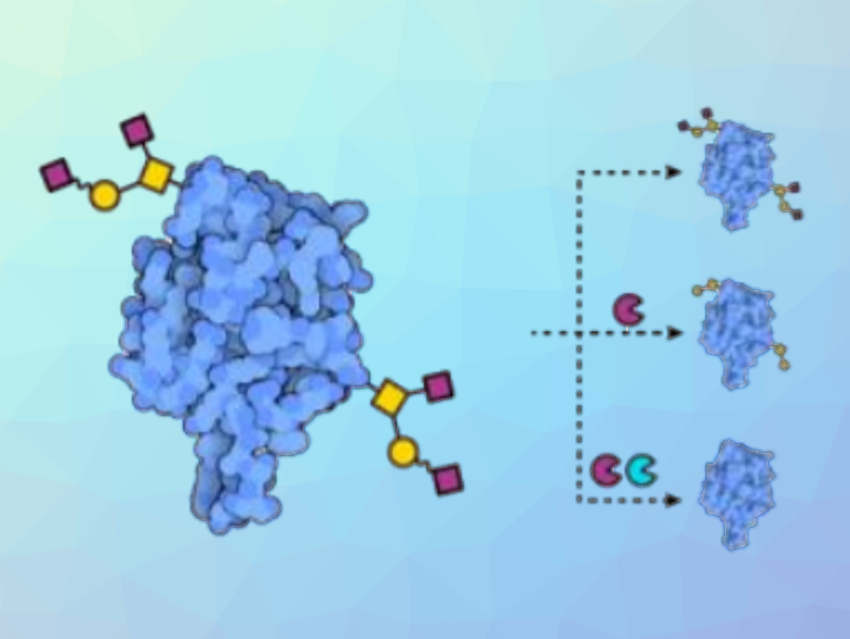
Glycans are the sugars that are attached to the surface of glycoproteins. The number and types of these sugars are often highly variable, and it can be challenging to assess their structures and impact on protein stability. However, they play critical roles in ensuring that proteins are properly folded and in dictating interactions with other molecules.
There is, for example, little information about the effects of glycosylation on the immune scavenger protein DC-SIGN (dendritic cell‐specific intercellular adhesion molecule‐3 grabbing non‐integrin). DC-SIGN has been implicated as an “infection enhancer” in previous reports of coronavirus epidemics. It is thought to enhance the circulation of viral particles through interactions with viral glycans via its carbohydrate recognition domain (CRD).
Carol V. Robinson, Weston B. Struwe, and colleagues, University of Oxford, UK, have used high-resolution native mass spectrometry (MS) experiments to study the effects of glycosylation on a DC-SIGN protein. The team expressed and purified the DC‐SIGN CRD using human embryonic kidney (293T) cells. Mass spectra then showed a range of possible mono- and disaccharides bound to the protein.
To distinguish sugars with equal masses, the team used enzymatic digestions specific for the type and linkage of individual monosaccharides to remove known types of sugars (pictured). After the enzymatic reactions, they recorded new mass spectra and were, thus, able to determine the structure of the O-glycans. The approach revealed O-glycans on the CRD of DC-SIGN that could influence interactions with glycans found on viral spike proteins.
- Correlating Glycoforms of DC-SIGN with Stability Using a Combination of Enzymatic Digestion and Ion Mobility Mass Spectrometry,
Hsin-Yung Yen, Idlir Liko, Joseph Gault, Di Wu, Weston B. Struwe, Carol V. Robinson,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202005727