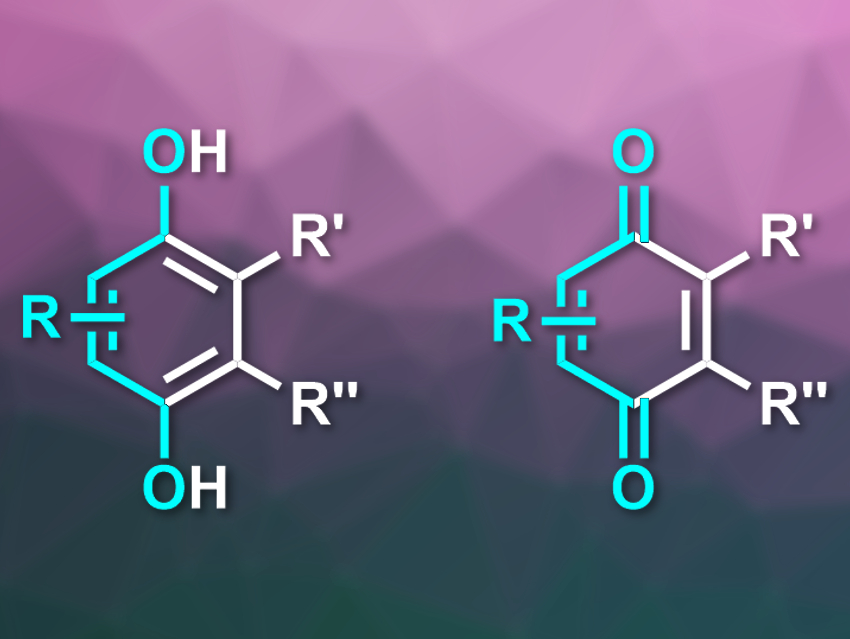
The hydroquinone–benzoquinone redox system is essential for life. Benzoquinones (example pictured on the right) act as electron carriers in cellular respiration and in the light reaction in photosynthesis. Hydroquinones (example pictured on the left) and benzoquinones are also part of many pharmaceuticals, including important anticancer drugs.
However, the synthesis of the hydroquinone motif, especially if it is embedded in a more complex molecular network, can be cumbersome and often involves many synthetic steps. Christopher G. Newton, The University of Adelaide, Australia, and colleagues have found an elegant shortcut. Using furans as Diels–Alder diene equivalents, they succeeded in forming the para-quinone ring system in a one-pot Diels–Alder/ring-opening/tautomerization process.
The Diels–Alder Reaction
The Diels–Alder reaction, discovered in the late 1920s, is famous for its ability to create six-membered carbon rings in a one-step cycloaddition process. Usually, an electron-rich diene reacts with a dienophile, which is an electron-deficient alkene, to form a substituted cyclohexene. Over the decades, thousands of different dienes and dienophiles have been used to prepare a vast array of rings, often as a key step in the synthesis of natural products with complex polycyclic frameworks.
In contrast to this simple path to six-membered rings, the traditional approaches to functionalized para-hydroquinones (pictured above on the left) either start from benzenes, which are first functionalized and then selectively oxidized in the 1,4-positions, or from a protected hydroquinone, which is functionalized in later steps.
The para-Quinone System
Many secondary metabolites and polyphenols contain the para-hydroquinone motif. These naturally occurring products are promising pharmaceuticals, for example, the anti-inflammatory lucidumone or the neuroprotective agent indanostatin. Hydroquinones are also found in some well-established pharmaceuticals, such as the chemotherapy drug doxorubicin.
In their attempt to shorten lengthy functionalization strategies, Newton and his team hypothesized that the hydroquinone could be the product of a [4+2] cycloaddition between a vicinal bisketene—a molecule with two unstable ketene (–C=C=O) end groups— and a substituted alkene. The resulting cyclohexenedienone would then undergo a double keto–enol tautomerization to give the 1,4-hydroquinone.
The Vicinal Bisketene Approach
However, a vicinal bisketene is not something commonly found lying around on the lab bench! With this in mind, the researchers developed a reasonably stable bisketene equivalent, which is a furan substituted at the 2,5-positions by bulky butyldimethylsilyloxy groups. The team added that these and other furan derivatives suitable in the reaction were readily accessible from their corresponding cycloanhydrides.
Having identified silyloxy-substituted furans as diene equivalents, the researchers found that they reacted with many of the conventional Diels–Alder dienophiles to form to the desired hydroquinone products, even the less reactive ones. However, according to the team, both reaction conditions and solvents had to be optimized in each case.
As a further step, the team also explored acetylenic derivatives and benzynes for their use as dienophiles to make it possible to synthesize even benzoquinones—oxidized derivatives of hydroquinones (pictured above on the right) and important oxidation agents. The benzynes were generated from their respective ortho-iodotriflates as precursors.
Step-Economic Synthesis
Using furans as diene equivalents in the Diels–Alder reaction enabled the researchers to synthesize hydroquinones and benzoquinones in far fewer steps than is usually possible. As proof of concept, they prepared the novel neuroprotective agent indanostatin in three steps on a gram scale, while the originally reported route involved nine steps and gave a smaller yield [1].
The researchers also replaced the silyloxylated furans with silyloxylated pyrroles, which have a nitrogen atom in the aromatic five-membered ring instead of the oxygen, and they established a cycloaddition pathway leading directly to paracetamol derivatives.
- Bisketene Equivalents as Diels–Alder Dienes,
Isuru Dissanayake, Jacob D. Hart, Emma C. Becroft, Christopher J. Sumby, Christopher G. Newton,
J. Am. Chem. Soc. 2020.
https://doi.org/10.1021/jacs.0c06306
Reference
- [1] Annulations of 5-Phenylthiobutenolides and First Synthesis of (±)-Indanostatin,
Shuai Wang, George Kraus,
Synlett 2019, 30, 353–355.
https://doi.org/10.1055/s-0037-1611462