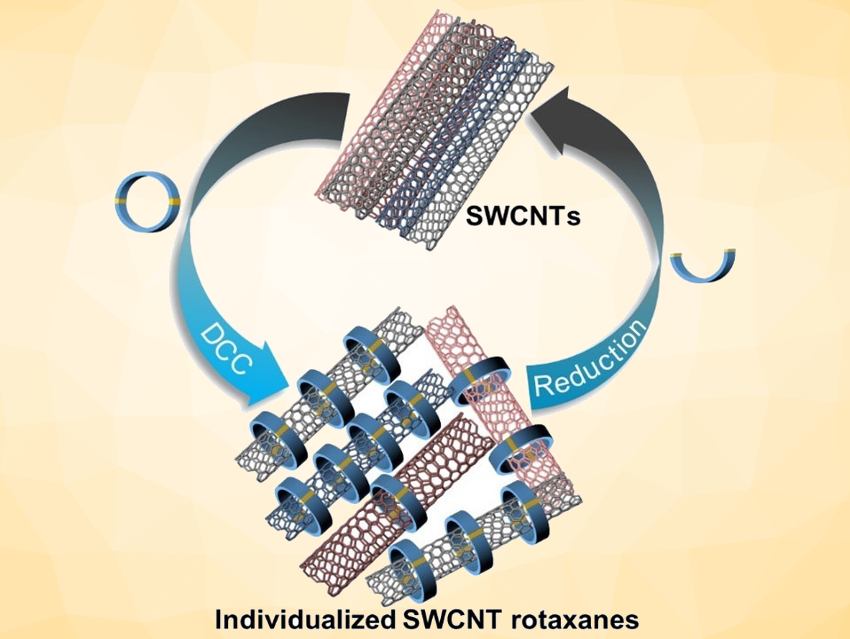
The functionalization and purification of single-walled carbon nanotubes (SWCNTs) under mild conditions is still challenging, which hampers their widespread use. One solution to the functionalization problem has been developed by Emilio M. Pérez, Universidad Complutense de Madrid, Spain, and colleagues, who “wrapped” discrete macrocycles around SWCNTs using irreversible ring-closing olefin metathesis [1]. This essentially turns the nanotubes into poly[n]rotaxanes.
Dirk M. Guldi, Friedrich-Alexander University Erlangen-Nürnberg, Erlangen, Germany, Max von Delius, University of Ulm, Germany, and colleagues have extended this approach to disulfide-based macrocycles, which can be formed in a reversible reaction. The team designed and synthesized u-shaped building blocks for the macrocycles that contain a curved π-system with a thiol group at either end. SWCNTs can act as templates for the formation of macrocycles from these u-shaped building blocks, leading to the desired mechanically interlocked compounds.
SWCNTs were suspended in dimethylformamide (DMF) by sonication and the preformed macrocycles as well as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and dithiothreitol (DTT) were added. Under these conditions, the disulfide bonds dynamically break and close, and the macrocycles can open and re-form around the nanotubes. The reaction was then quenched and the products collected by filtration. The structure of the mechanically interlocked products (see the animation below) was confirmed by UV/Vis/NIR-, Raman-, photoluminescence excitation (PLE)- and transient absorption spectroscopy as well as high-resolution transmission electron microscopy (HR-TEM) studies.
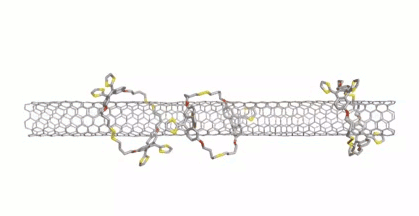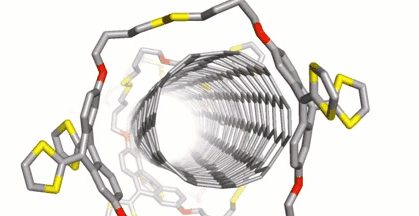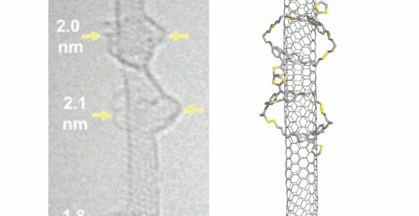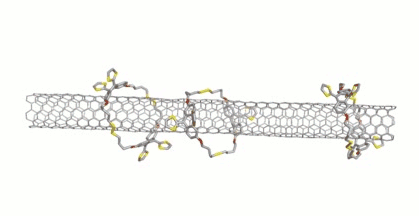
Image credit: VH Shyamkumar
Due to the reversible formation of the disulfide-based macrocycles, they can be cleaved again under mild, reductive conditions. Small SWCNTs are predominantly functionalized by smaller macrocycles, whereas the larger SWCNTs match larger macrocycles. This could be useful to purify mixtures of SWCNTs based on a size-match between macrocycle and nanotube.
- Dynamic covalent formation of concave disulfide macrocycles mechanically interlocked with single‐walled carbon nanotubes,
Bugga Balakrishna, Arjun Menon, Kecheng Cao, Sebastian Gsänger, Sebastian B. Beil, Julia Villalva, Oleksandr Shyshov, Oliver Martin, Andreas Hirsch, Bernd Meyer, Ute Kaiser, Dirk M. Guldi, Max von Delius,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202005081
Reference
- [1] Mechanically Interlocked Single-Wall Carbon Nanotubes,
Alberto de Juan, Yann Pouillon, Luisa Ruiz-González, Almudena Torres-Pardo, Santiago Casado, Nazario Martín, Ángel Rubio, Emilio M. Pérez,
Angew. Chem. Int. Ed. 2014, 53, 5394–5400.
https://doi.org/10.1002/anie.201402258