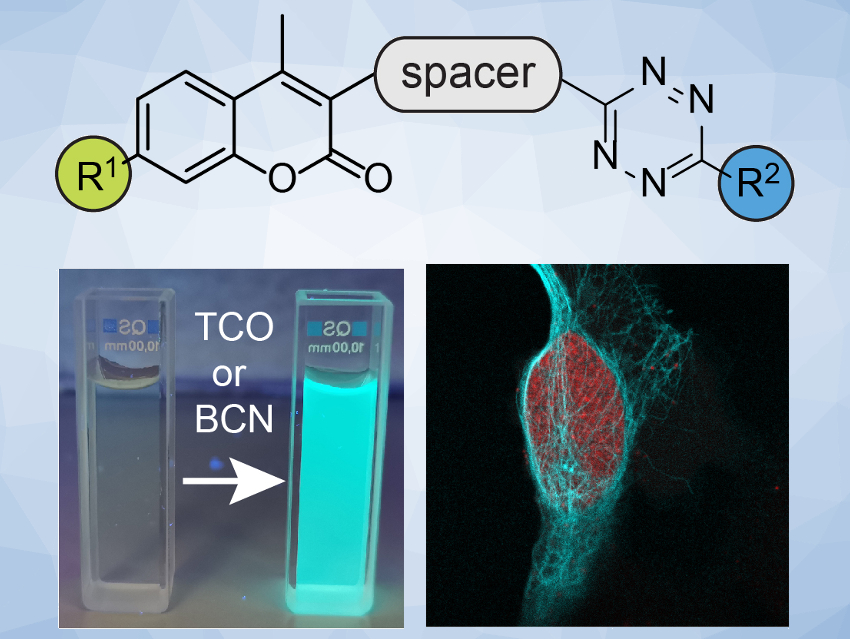
The labeling and visualization of biomolecules within living systems are important for the study of complex biological processes. Commonly used strategies for this are based on fluorescent dyes that are attached to enzymes or proteins by a selective chemical click reaction and then observed by fluorescence microscopy. One problem in such experiments is the generation of unspecific signals arising from an excess of the fluorescent probe. Fluorescent probes that only light up upon a specific chemical reaction can solve this problem and allow improved precision in the observation of biological systems.
Milan Vrabel and colleagues, Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague, have systematically studied the fluorogenic properties of a series of coumarin–tetrazine probes (pictured above). These probes produce fluorescent dyes after a reaction with a trans-cyclooctene (TCO) or bicyclononyne (BCN) dienophile. The team prepared a variety of probes starting from a coumarin derivative. They used a reaction sequence that includes an amination to introduce R1, a Suzuki coupling to introduce a (hetero-)aromatic spacer, and a cycloaddition to form the R2-substituted tetrazine unit.
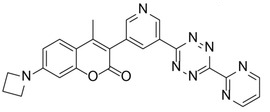 The team found that azetidine or N,N-dimethylpiperazine groups attached to the coumarin scaffold lead to improved fluorescence quantum yield. They identified a probe with an azetidine-substituted coumarin, a pyridyl linker, and a 6-pyrimidine‐substituted tetrazine (pictured on the right) as the most versatile probe with superior labeling efficiency in live cells. The team attributes the probe’s good performance to a combination of good reaction kinetics and cell permeability.
The team found that azetidine or N,N-dimethylpiperazine groups attached to the coumarin scaffold lead to improved fluorescence quantum yield. They identified a probe with an azetidine-substituted coumarin, a pyridyl linker, and a 6-pyrimidine‐substituted tetrazine (pictured on the right) as the most versatile probe with superior labeling efficiency in live cells. The team attributes the probe’s good performance to a combination of good reaction kinetics and cell permeability.
- A Systematic Study of Coumarin–Tetrazine Light‐Up Probes for Bioorthogonal Fluorescence Imaging,
Juraj Galeta, Rastislav Dzijak, Jan Obořil, Martin Dračínský, Milan Vrabel,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.202001290