1,3,5-Trisubstituted cyclohexane derivatives can be used as synthetic platforms in, e.g., organic, supramolecular, and coordination chemistry. The geometrical structure of such synthetic platforms directly influences the structure and properties of the final molecules or materials. Tripodal scaffolds such as 1,3,5-trisubstituted cyclohexanes are widely used in molecular and supramolecular design. However, the derivatization of the cyclic backbone in these platforms is challenging and generally requires multistep synthetic routes.
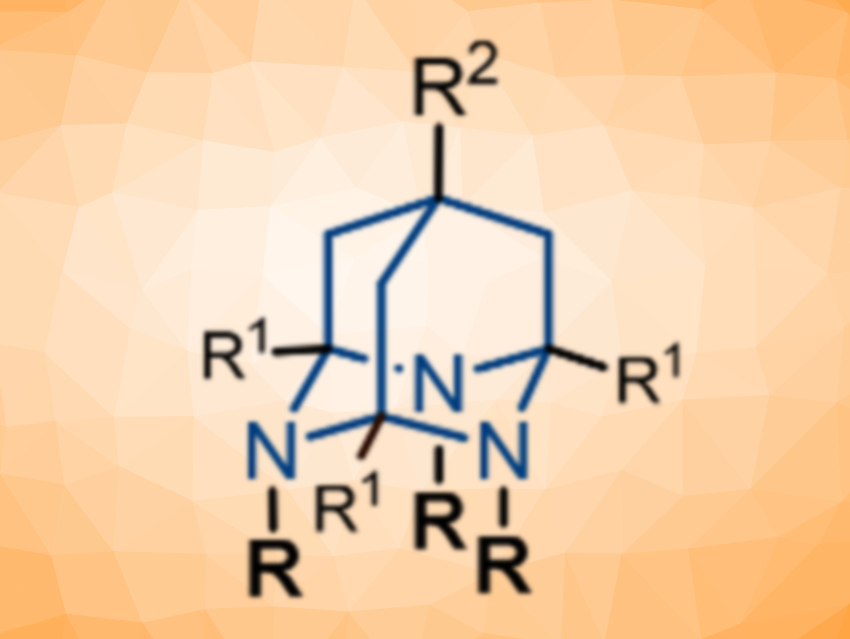
Artem N. Semakin, Zelinsky Institute of Organic Chemistry, Moscow, Russia, Alexey Yu. Sukhorukov, Zelinsky Institute of Organic Chemistry and Plekhanov Russian University of Economics, Moscow, and colleagues have designed new versatile 3D synthetic platforms based on the 2,4,9-triazaadamantane (TRIAD) scaffold (pictured above). TRIADs contain a stabilized 1,3,5-triazacyclohexane ring, which can be easily derivatized at the nitrogen atoms by using “clickable” groups. These heterocages were synthesized from triallylmethane derivatives (pictured below) via an ozonolysis that gives tris-carbonyl compounds. The carbonyl intermediates were then reacted with N-nucleophiles to give the desired cages.
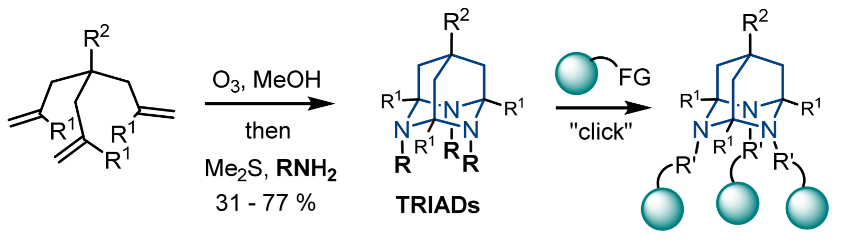
The researchers introduced reactive groups at the TRIADs’ nitrogen atoms during cage formation, e.g., propargyl groups that can be used in copper-catalyzed azide‐alkyne coupling (CuAAC) click reactions. The team also prepared tris-amino-substituted cages, which can react with aldehydes to give tris-hydrazones. A tri-hydroxy-substituted cage was synthesized and reacted with phenylboronic acid to form the corresponding boronate adduct. These three different click-like modification strategies show the chemical versatility of the developed 3D scaffold.
- 2,4,9-Triazaadamantanes with “Clickable” Groups: Synthesis, Structure and Applications as Tripodal Platforms,
Artem Semakin, Yulia Nelyubina, Sema Ioffe, Alexey Sukhorukov,
Eur. J. Org. Chem.y 2020.
https://doi.org/10.1002/ejoc.202000832