Separation of Trace Acetylene from Ethylene
Ethylene, a key feedstock in the chemical industry, often includes traces of acetylene contaminants, which need to be removed. Dan Zhao, National University of Singapore, and colleagues have found a robust and regenerable porous metal–organic framework (MOF) that captures acetylene selectively and with extraordinary efficiency. Its synergistic combination of tailor-made pore sizes and chemical docking sites makes the material especially efficient.
Ethylene is the most important chemical precursor for ethanol and polyethylene and is mainly produced by steam cracking. Although the ethylene fraction is usually very pure (more than 99 %), remaining traces of acetylene contaminants can destroy the catalysts used in downstream processes.
As ethylene and acetylene are very similar and only differ in the amount of hydrogen atoms, the separation of both gases is elaborate and difficult. The current industrial processes rely on distillation, which consumes a huge amount of energy.
Ultramicroporous Metal–Organic Frameworks
However, hydrocarbon compounds can bind to porous substances such as MOFs. MOFs are made of metal ions and organic ligands. They contain pores and chemical docking sites that can be designed to capture specific molecules from a stream of gas at ambient conditions. However, for the separation of ethylene and acetylene, the industry demands robust, regenerable, highly selective, and cheap materials, which had not been found so far.
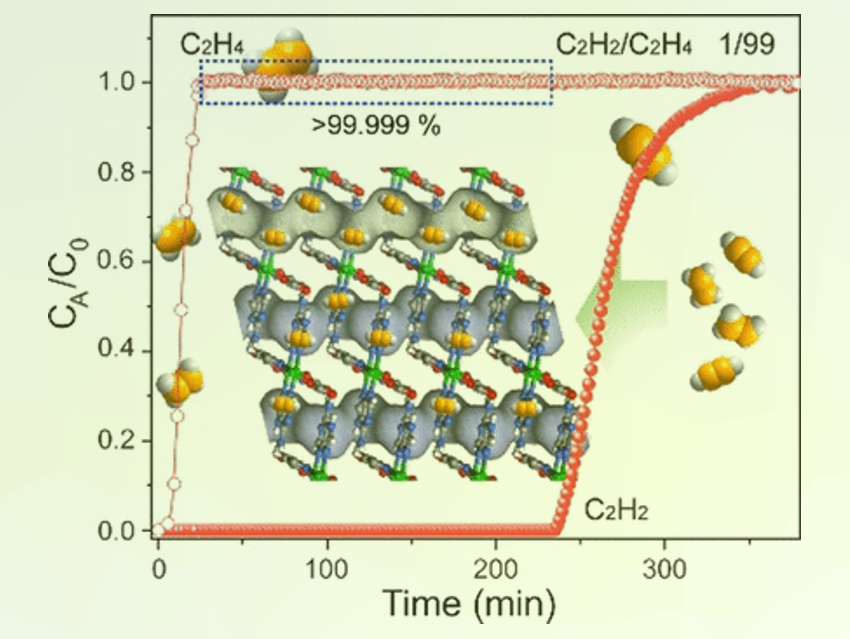
The researchers have developed a MOF specific for acetylene capture that may meet the demands of extraordinary selectivity and robustness. They focused on an established MOF with nickel sites, but “opened up” these nickel sites for the binding of more molecules by activating them and exposing them to the pores so that they were able to bind two guest molecules at once.
In addition, the team adjusted the pore sizes of the MOF to allow entry only for very small gas molecules, and filled the pore walls with chemical groups that would attract acetylene over ethylene through stronger electrostatic and chemical interactions.
Synergy of Pore Structure and Size
Thus, combining small pore sizes with the open nickel sites and sites for preferential acetylene binding, the researchers have created a Ni-MOF called Ni3(pzdc)2(7 Hade)2 that is extraordinarily selective, robust, stable, and can be regenerated. According to the study, the Ni-MOF purified the ethylene stream by a factor of a thousand and kept the selectivity high across a range of pressures and regeneration cycles. In addition, the Ni-MOF can be prepared in a standard hydrothermal procedure.
The team points out that the synergy of pore geometry and size, combined with chemical interactions, can be further enhanced and may lead to even more effective separations. This is interesting for industrial applications.
- Efficient Trapping of Trace Acetylene from Ethylene in an Ultramicroporous Metal‐Organic Framework via Synergistic Effect of High‐Density Open Metal Sites and Electronegative Sites,
Zhaoqiang Zhang, Shing Bo Peh, Yuxiang Wang, Chengjun Kang, Weidong Fan, Dan Zhao,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202009446