Microdroplets can act as chemical reactors and accelerate reactions at the air/solution interface. They are useful in electrochemical synthesis—particularly, for transformations of C(sp3)–H, C(sp2)–H, C=C, –OH, and C=O groups that generally react slowly. However, maintaining contact between the microdroplets and the electrode is challenging.
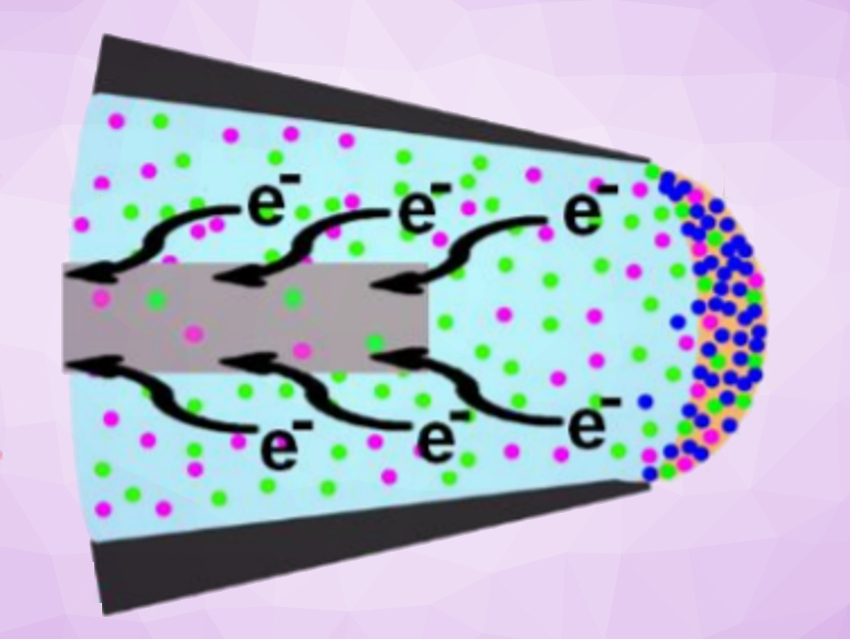
Xin Yan, Texas A&M University, USA, and colleagues have developed an interfacial microreactor that can accelerate electrochemical reactions that are not observed in conventional electrochemical cells. The microreactor is formed at the solution/air interface in an electrospray emitter. The spray voltage is tuned to form a meniscus that has a large surface area (pictured).
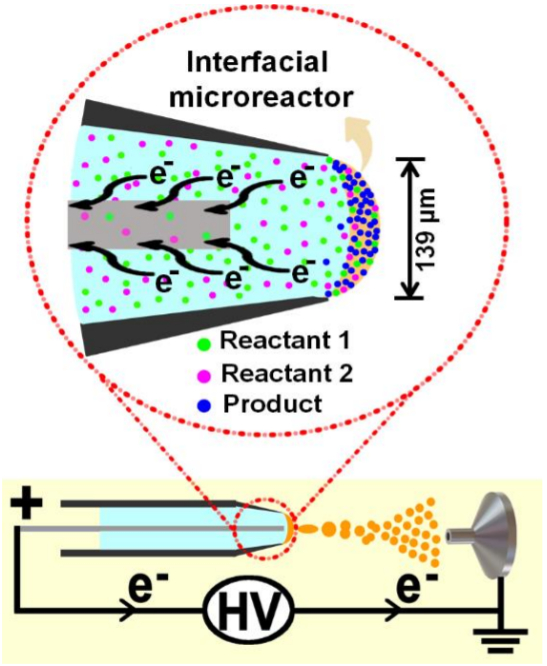
The reactants at or near the meniscus receive or lose electrons to or from the electrode upon application of voltage, and the reaction products are immediately formed at the interface. The researchers performed an electrooxidative C–H/N–H coupling of N,N’-dimethylaniline and phenothiazine, and an electrochemical derivatization of benzyl alcohol. They achieved acceleration factors of 67 and 111, respectively, compared with the corresponding bulk reactions.
- Accelerating Electrochemical Reactions in a Voltage‐Controlled Interfacial Microreactor,
Heyong Cheng, Shuli Tang, Tingyuan Yang, Shiqing Xu, Xin Yan,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202007736