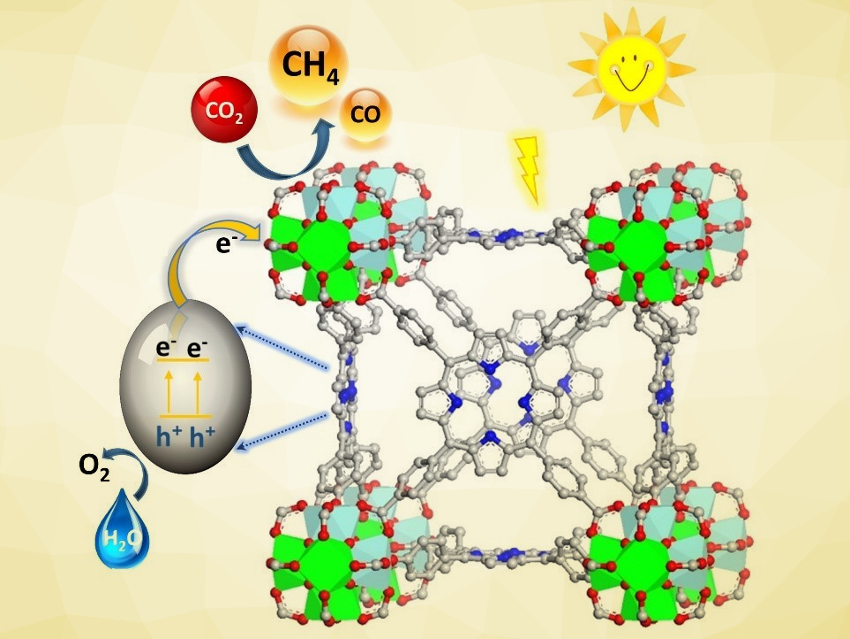
Photosynthesis, which uses sunlight to convert CO2 and H2O into carbohydrates and O2, is the prime example of a reaction that can produce chemical fuels from waste CO2 and mitigate climate change. Artificial photosynthesis aims to achieve similar CO2 photoreduction reactions using chemical systems. Metal–organic frameworks (MOFs), for example, can promote CO2 photoreduction in the liquid phase, but usually require organic reagents as the electron donor.
Babu Joseph, University of South Florida, Tampa, USA, Shengqian Ma, University of North Texas, Denton, USA, and colleagues have synthesized a mixed-metal (Ti/Zr) porphyrinic MOF (pictured) that can promote CO2 photoreduction in the gas phase without organic sacrificial reagents. The team started from the zirconium-based MOF-525, which is composed of tetrakis(4‐carboxyphenyl)porphyrin (H4TCPP) linkers and Zr6O4(OH)4 units. They used a metal metathesis procedure to partially replace Zr with Ti, resulting in a Ti/Zr atomic ratio of 9:1. The structure of the MOF was retained after the exchange.
The researchers then performed CO2 photoreduction experiments using the mixed-metal MOF as the catalyst in the presence of water vapor under irradiation with visible light. The MOF reduced CO2 into CH4, with high selectivity toward the formation of CH4 over CO. The MOF uses the porphyrin units to absorb visible light, the mixed-metal clusters act as reactive sites, and water vapor is used as an electron donor. This first example could be a basis for the development of other MOF‐based photocatalysts for gas-phase CO2 reduction that do not require organic sacrificial reagents.
- A Mixed-Metal Porphyrinic Framework Promoting Gas-Phase CO2 Photoreduction without Organic Sacrificial Agents,
Wen-Yang Gao, Huong T. Ngo, Zheng Niu, Weijie Zhang, Yanxiong Pan, Zhongyu Yang, Venkat R. Bhethanabotla, Babu Joseph, Briana Aguila, Shengqian Ma,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202001610