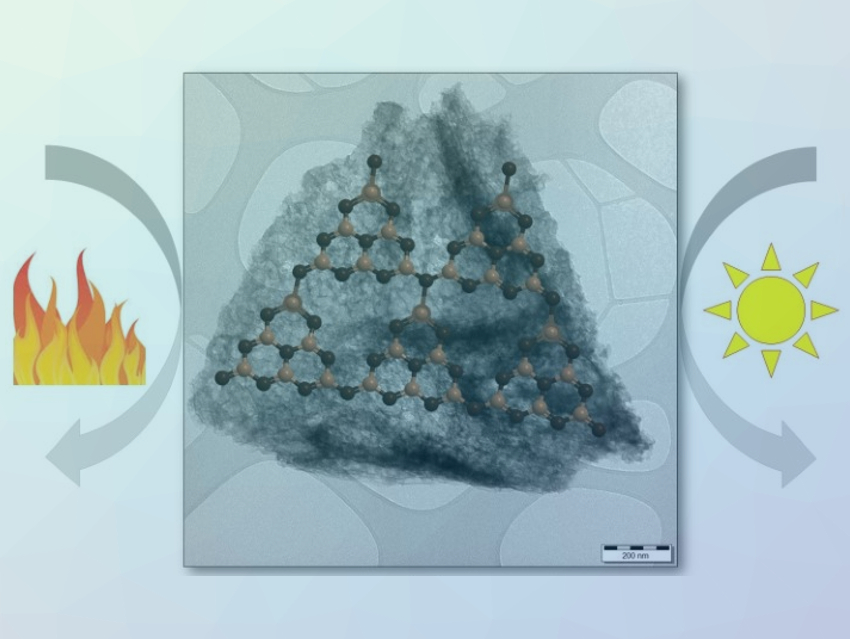
Graphitic carbon nitride (g-C3N4), a layered polymeric semiconductor material, is a promising catalyst due to its abundance, nontoxicity, and stability. It has possible applications in thermal, photo-, and electrophotocatalysis. However, pristine g-C3N4 suffers from a limited photocatalytic activity and a low surface area. Thus, an effective process for the chemical doping of g-C3N4 and for the generation of g-C3N4-based materials with a large surface area would be useful.
Manoj B. Gawande, Palacký University Olomouc, Czech Republic, and Institute of Chemical Technology, Jalna, India, Radek Zbořil, Palacký University Olomouc, and colleagues have developed new P- and F-co-doped amorphous carbon nitride materials. The carbon nitride derivatives were synthesized via a sol-gel-mediated thermal condensation of dicyandiamide and 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) in the presence of hexadecyltrimethylammonium bromide (CTAB) and tetraethyl orthosilicate (TEOS) at 550 °C. The synthesized material has a mesoporous structure, shows good visible light absorption, and has a large surface area.
The doping with P- and F-atoms narrows the optical band gap and enables an excellent photocatalytic activity for the aqueous reduction of carbon dioxide into methanol under visible-light irradiation. The activity is fifteen times higher than that of pure carbon nitride. The doping also turns the material into an acid-base bifunctional catalyst that is suitable for the thermal conversion of carbohydrates (glucose and xylose) into high-value furanic compounds (5-hydroxymethyl-2-furaldehyde (HMF) and furfural).
- Mesoporous P‐ and F‐co‐doped Amorphous Carbon Nitride: Nanocatalysts for Photocatalytic CO2 Reduction and Thermocatalytic Furanics Synthesis from Sugars,
Subodh Kumar, Manoj B. Gawande, Josef Kopp, Štěpán Kment, Rajender S. Varma, Radek Zboril,
ChemSusChem 2020.
https://doi.org/10.1002/cssc.202001172