Dinitrogen (N2) and dioxygen (O2) are the main components of air. The activation of N2 and O2 is an interesting challenge for chemists. The industrial conversion of N2 to ammonia, for example, is performed on a large scale using the Haber–Bosch process, but requires very high temperatures and pressures. Uranium-based materials can act as effective catalysts for ammonia production from N2, but the cleavage of N2 or O2 by molecular uranium compounds under mild conditions had not been accomplished so far.
Congqing Zhu, Nanjing University, China, and colleagues have developed the first example of N2 and O2 cleavage by a uranium(III) complex under ambient conditions without an external reducing agent. The complex, [N(CH2CH2NPiPr2)3U]2(TMEDA) (TMEDA = tetramethylethylenediamine), was synthesized via the reduction of the uranium(IV) complex [N(CH2CH2NPiPr2)3UCl] with potassium graphite (KC8) in the presence of TMEDA under argon.
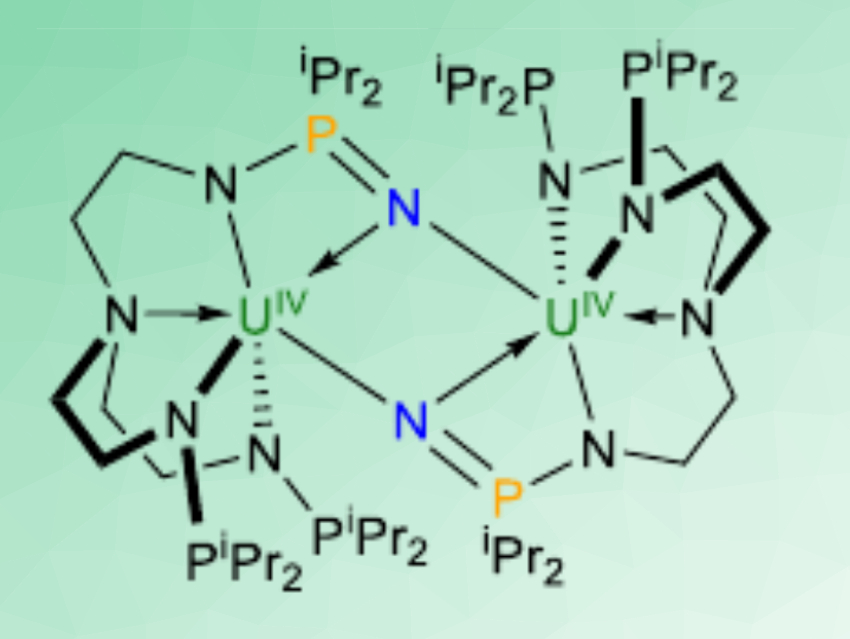
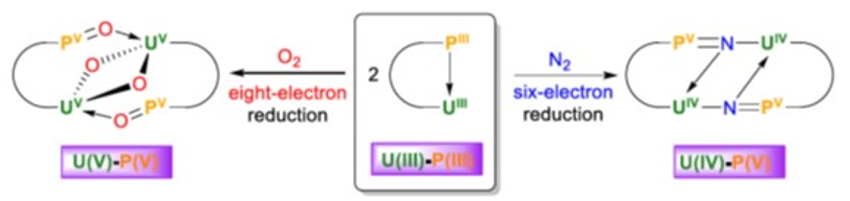
The complex can react with dinitrogen to give [N(CH2CH2NPiPr2)2(CH2CH2NPiPr2N)U]2 (pictured). The hydrolysis of this product gives ammonia. The uranium(III) complex can also react with dioxygen to give [N(CH2CH2NPiPr2)2(CH2CH2NPiPr2O)U]2(μ-O)2. Both reactions proceed under mild conditions (1 atm N2 or O2, room temperature). The ligand is non-innocent, i.e., it takes part in the reaction: electrons for the reduction of N2 and O2 are provided by both U(III) and P(III) atoms. The N2 triple-bond breaking implies a six-electron reduction, while the O2 cleavage involves an eight-electron reduction of two O2 molecules (schematically pictured below).
- Facile Dinitrogen and Dioxygen Cleavage by a Uranium(III) Complex: Cooperativity Between the Non-innocent Ligand and the Uranium Center,
Penglong Wang, Iskander Douair, Yue Zhao, Shuao Wang, Jun Zhu, Laurent Maron, and Congqing Zhu,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202012198