Mechanochemistry is a way of conducting chemical reactions by milling, stretching, shearing, pulling, swelling, or ultrasonication. In this approach, the mechanical energy allows molecules to overcome high energy barriers. While “traditional” mechanochemistry is based on energy-demanding processes and non-reversible constitutional changes, “soft” mechanochemistry relies on reversible conformational changes. When the mechanical stimulus is released, the initial state of the material can be restored. This can be used, e.g., to design mechano-sensitive probes.
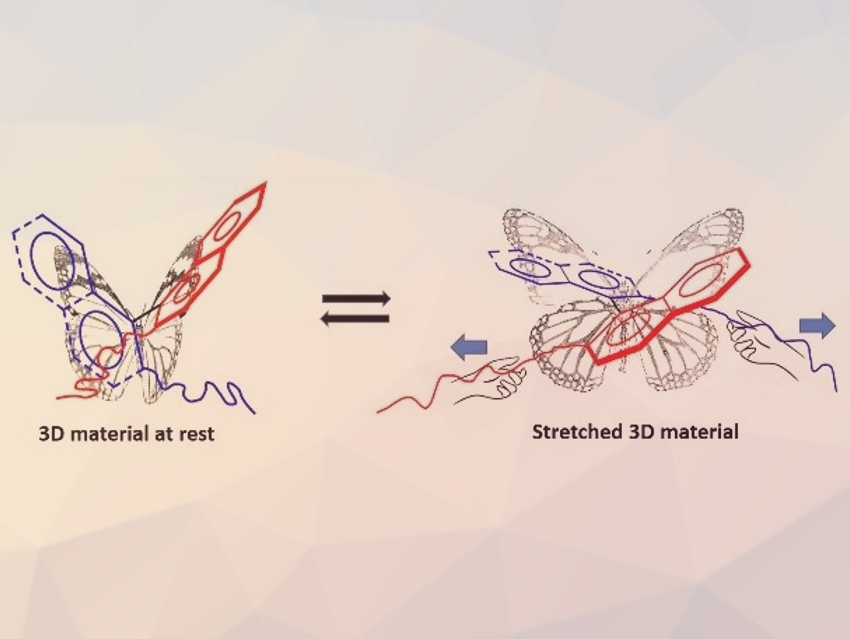
Loïc Jierry, Université de Strasbourg, France, and colleagues have used the concept of soft mechanochemistry to create an atropisomer crosslinked to a polymer architecture that changes its dihedral angle in a reversible and repeatable way (pictured) when a macroscopic stretching force is applied. These mechano-sensitive probes are composed of enantiopure binaphthol (BINOL) derivatives in polydimethylsiloxane (PDMS) as a host elastomer. The BINOL groups were incorporated into the polymer architecture using decenyl-chain linkers attached to the phenolic –OH groups.
The team measured circular dichroism (CD), linear dichroism (LD), and linear birefringence (LB) during mechanical deformations to investigate the effects on the polymer chains and the BINOL units. When the material was stretched up to ca. 170 %, the dihedral angle between the planes of the BINOL units increases continuously from ca. 110° to ca. 130°. Tuning the dihedral angle of axially chiral molecules by stretching might offer an original approach to modulate the chiroptical properties of atropisomer-containing materials.
- Reversible soft‐mechanochemical control of biaryl conformations through crosslinking in a 3D macromolecular network,
Julien B. Kelber, Amina Bensalah-Ledoux, Sarah Zahouani, Bruno Baguenard, Pierre Schaaf, Alain Chaumont, Stéphan Guy, Loïc Jierry,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202010604